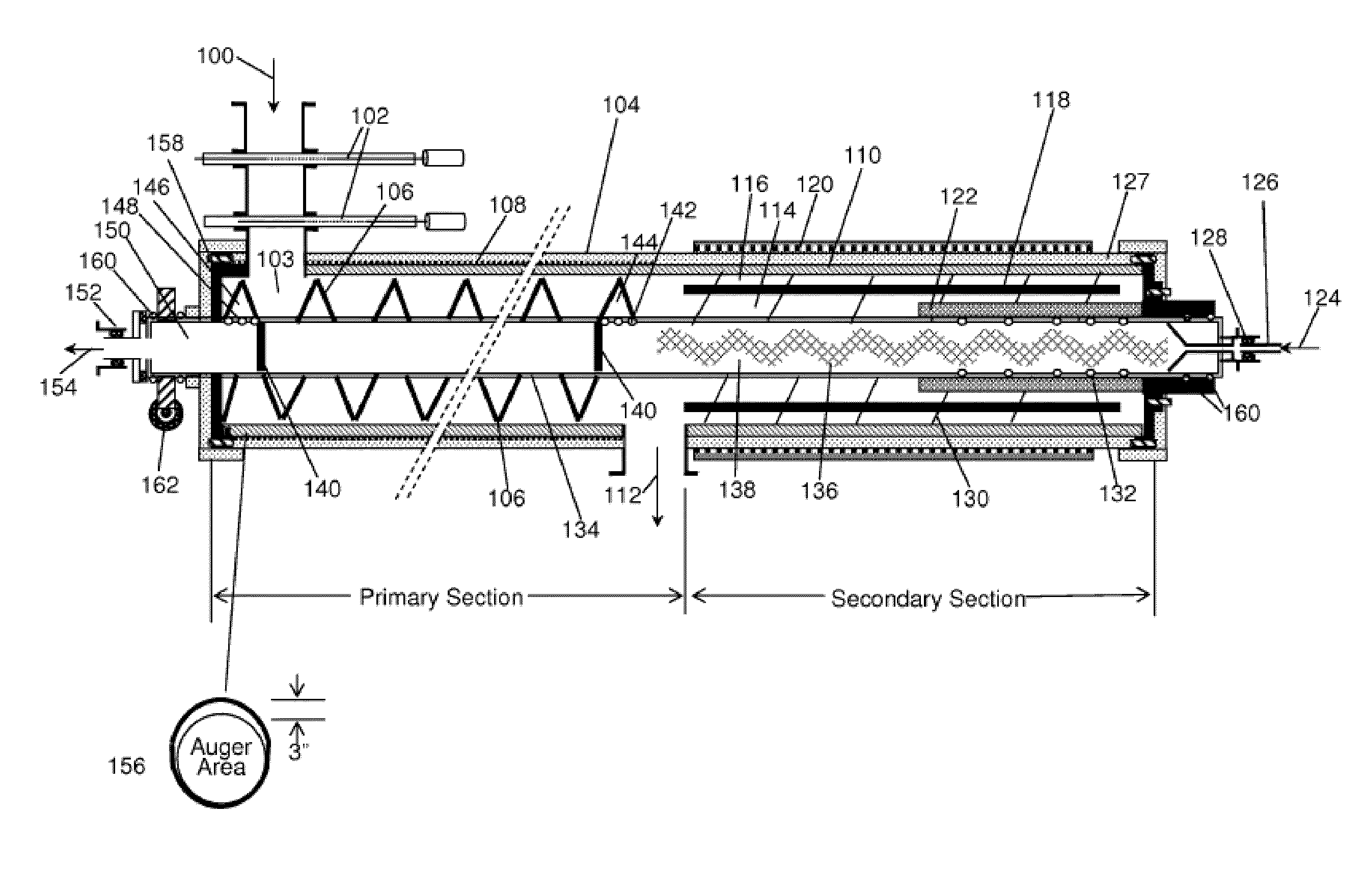
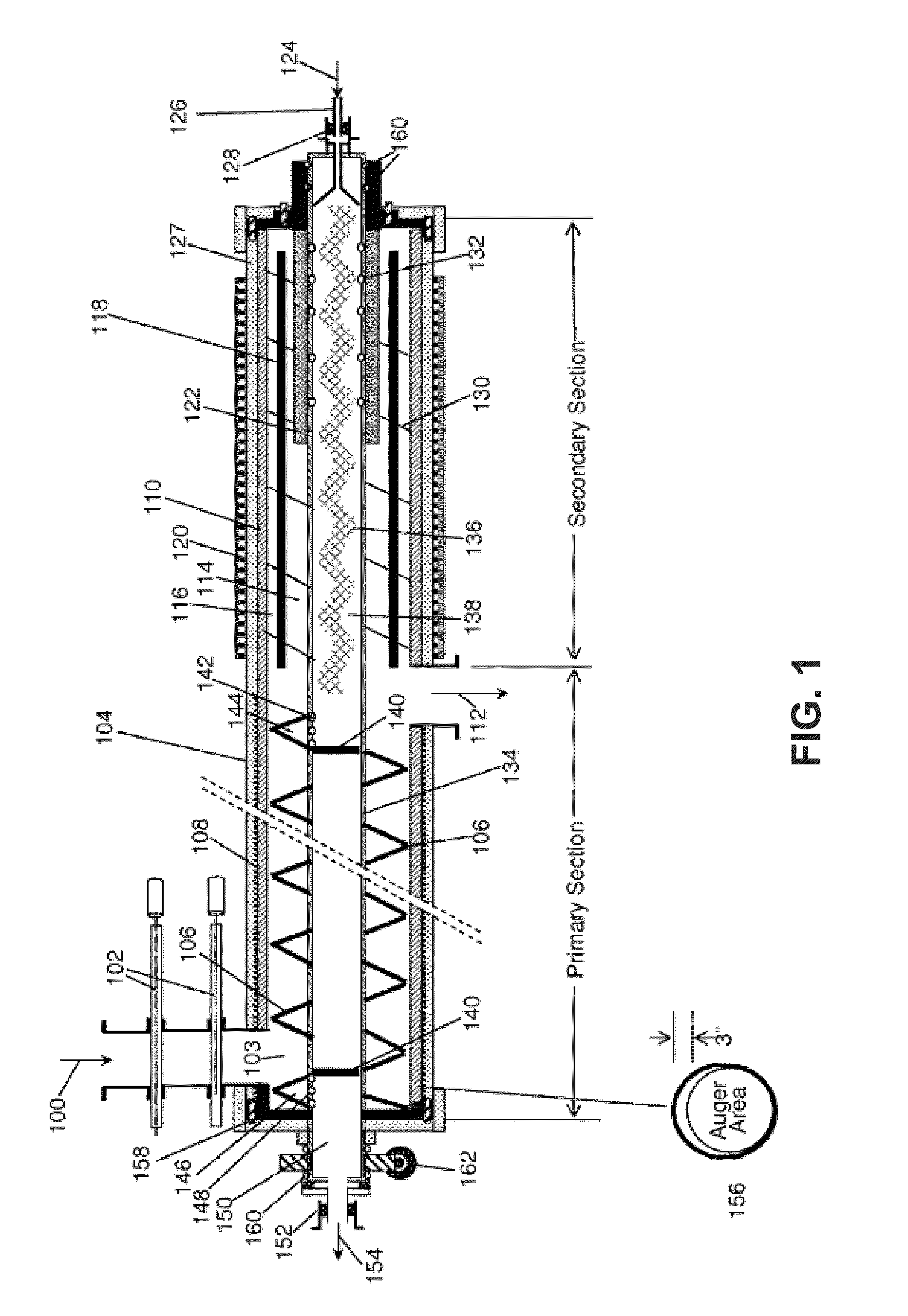
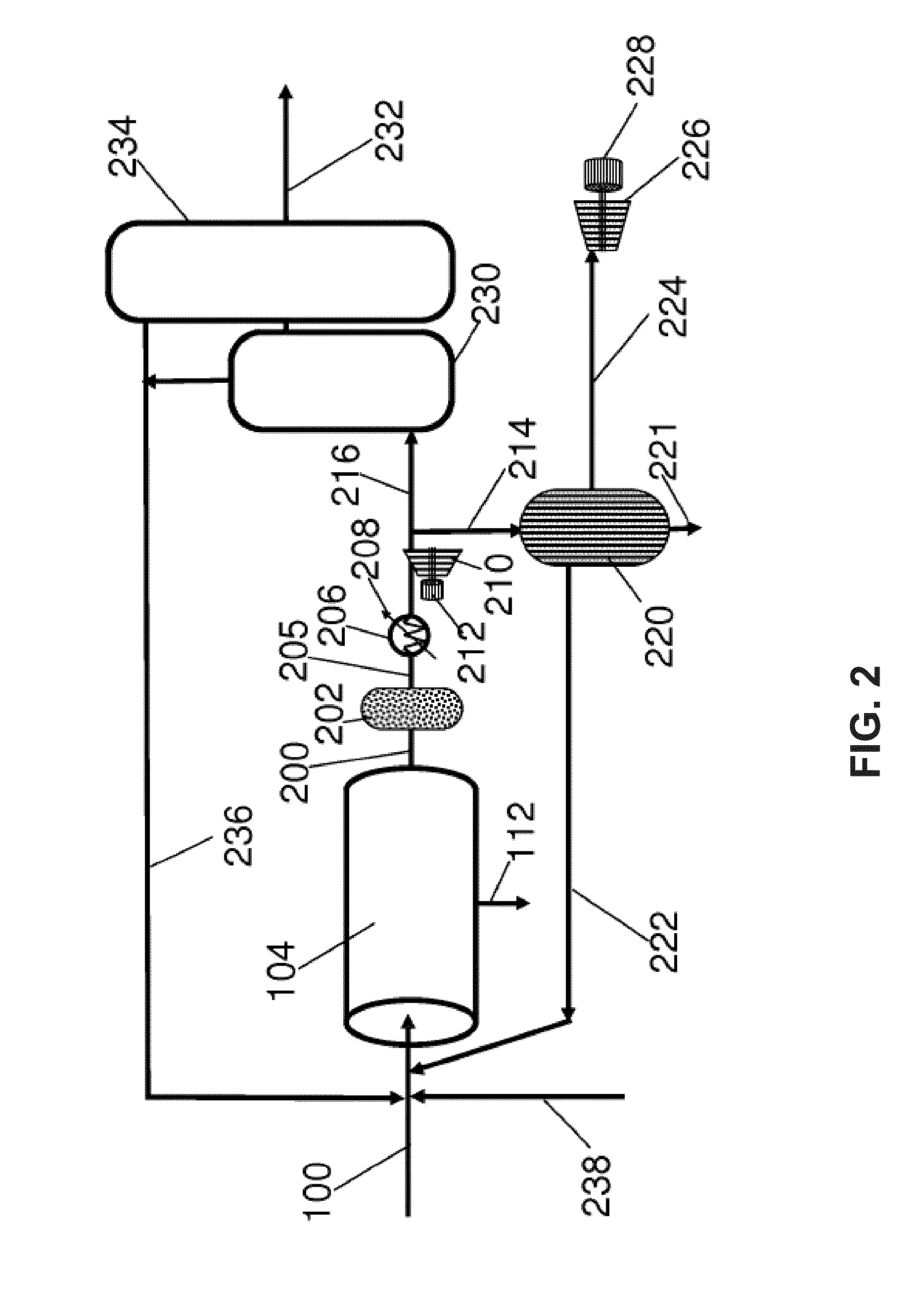
Process and system for converting waste to energy without burning
a technology of waste and energy conversion, applied in the direction of energy input, combustible gas production, combustible gas purification/modification, etc., can solve the problems of inefficient heat loss of process units, extreme sensitivity to various unknown chemical poisons, etc., and achieve the effect of large carbon dioxide emissions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example # 1
Example #1
Stoichiometric Steam
[0035]
C1H1.67O0.47+0.53H2O→CO+1.36H2
[0036]In this case 1 kg of waste will yield 1.45 kg of syngas.
example # 2
Example #2
Superstoichiometric in CO2 and C2H4
[0037]By contrast, here is the improved reforming reaction which involves a substoichiometric amount of steam but has the light hydrocarbon Fischer-Tropsch and shift / PSA overhead represented for simplicity by C2H4, plus CO2 and H2, added.
C1H1.67O0.47+0.55C2H4+0.69H2+1.5CO2+0.04H2O→3.68CO+2.67H2 [1]
[0038]In this case, 1 kg of waste will yield 5.11 kg of syngas, which is a very significant 350% increase in the mass of syngas product formed from a given mass of waste.
[0039]This achieves the formation only of CO and H2, and thus is stoichiometric which respect to the combination of steam plus CO2 plus C2H4. Thus, less steam (i.e. sub-stoichiometric) is required and greenhouse-problematic light hydrocarbons and CO2 can be used in large amounts to achieve overall the stoichiometric conversion to syngas desired with a preferred H2 / CO ratio around 0.73. CH4, C3H8 or other light hydrocarbons are actually involved in the real world in combination...
example # 3
Example #3
CO7 Enriched Syngas
[0049]A further improvement in the reforming reaction which involves a substoichiometric amount of steam but has the light hydrocarbon Fischer-Tropsch and shift / PSA overhead represented for simplicity by C2H4, plus CO2 and H2, added.
C1H1.67O0.47+0.2567C2H4+0.2CO2+1.434H2O→1.123CO+0.591CO2+3.029H2
[0050]In this case, the reformation reaction is allowed to form CO2 in the syngas, such that the stoichiometric ratio of (H2—CO2) / (CO+CO2)=1.42 which is favorable for the Fischer-Tropsch reaction as follows:
1.123CO+0.591CO2+3.029H2→0.0757C20H30+0.2CO2+1.904H2O
[0051]This achieves an increase in the amount of paraffin formed and greenhouse-problematic light hydrocarbons and CO2 are entirely recycle back into the reformer, with a small portion of the water condensed as product water. Thus, the use of Fischer-Tropsch is further simplified. As before, the CH4 is produced as the major part of the waste light gases coming off the tops of the Fischer-Tropsch gravity sep...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| temperatures | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com