Method and composition for treating osteoarthritis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Double Blind Randomized Trial in OA Canines
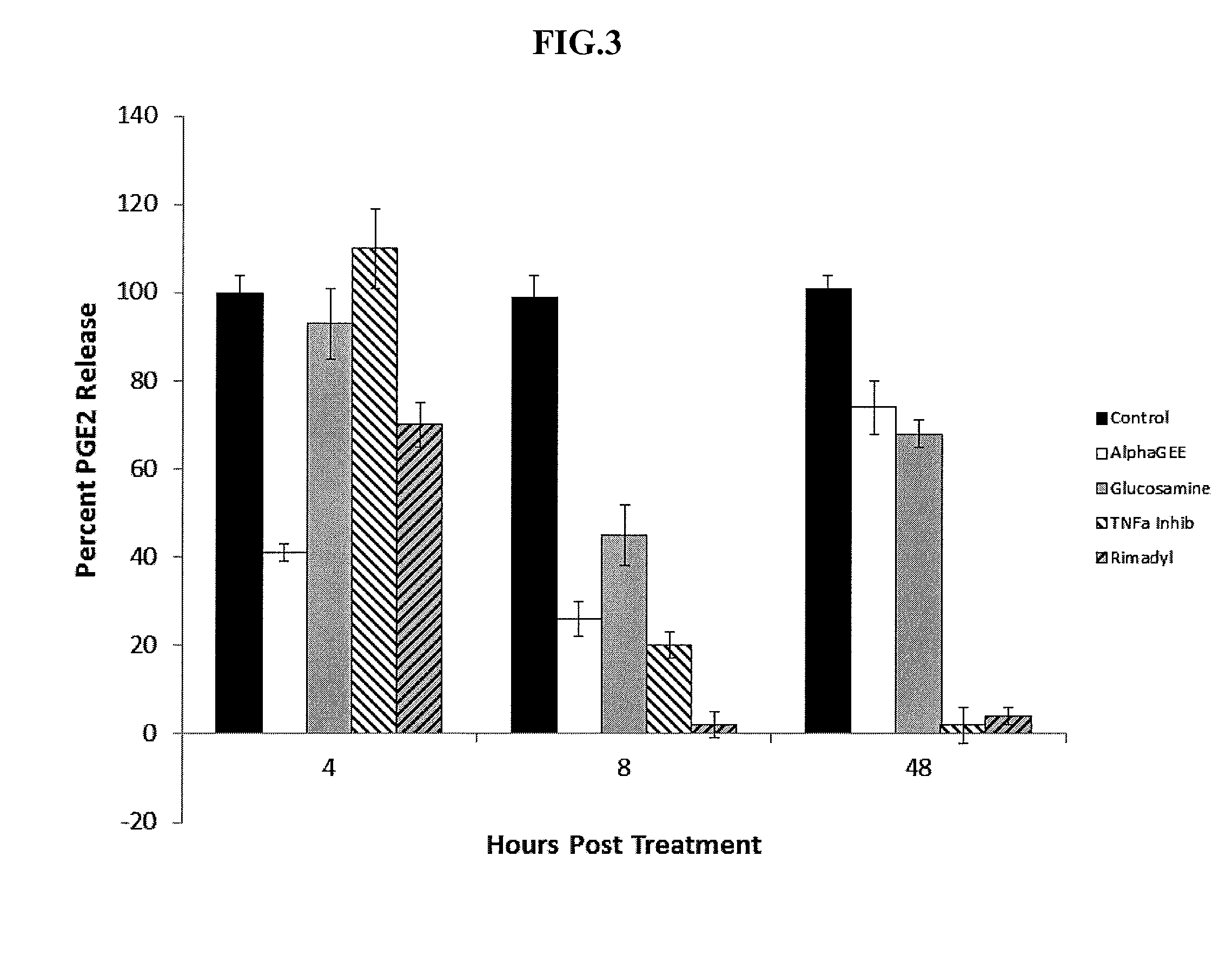
[0040]The following example evaluates the effectiveness of Alpha-GEE on both pain and mobility in a double-blind randomized trial in canines having OA. Results of the study indicate that evaluation of pain and mobility during the course of the trial demonstrated a significant improvement in canines receiving Alpha-GEE compared to canines in the placebo group with score reductions of approximately 50 percent versus 15 percent, respectively. Furthermore, while pedometer measurements showed no significant changes in the mobility of canines in the placebo group, the canines treated with Alpha-GEE showed an approximately 30 percent increase in physical activity during the two-week trial period. No significant differences were observed in prostaglandin E2 (“PGE2”) plasma levels during the trial period in either treatment group; however serum amyloid A (“SAA”) levels were lower in canines in the Alpha-GEE treatment group compared to placebo at the...
example 2
Effects of Alpha-GEE and Ibuprofen on the Release of PGE2 in Brain Endothelial Cells
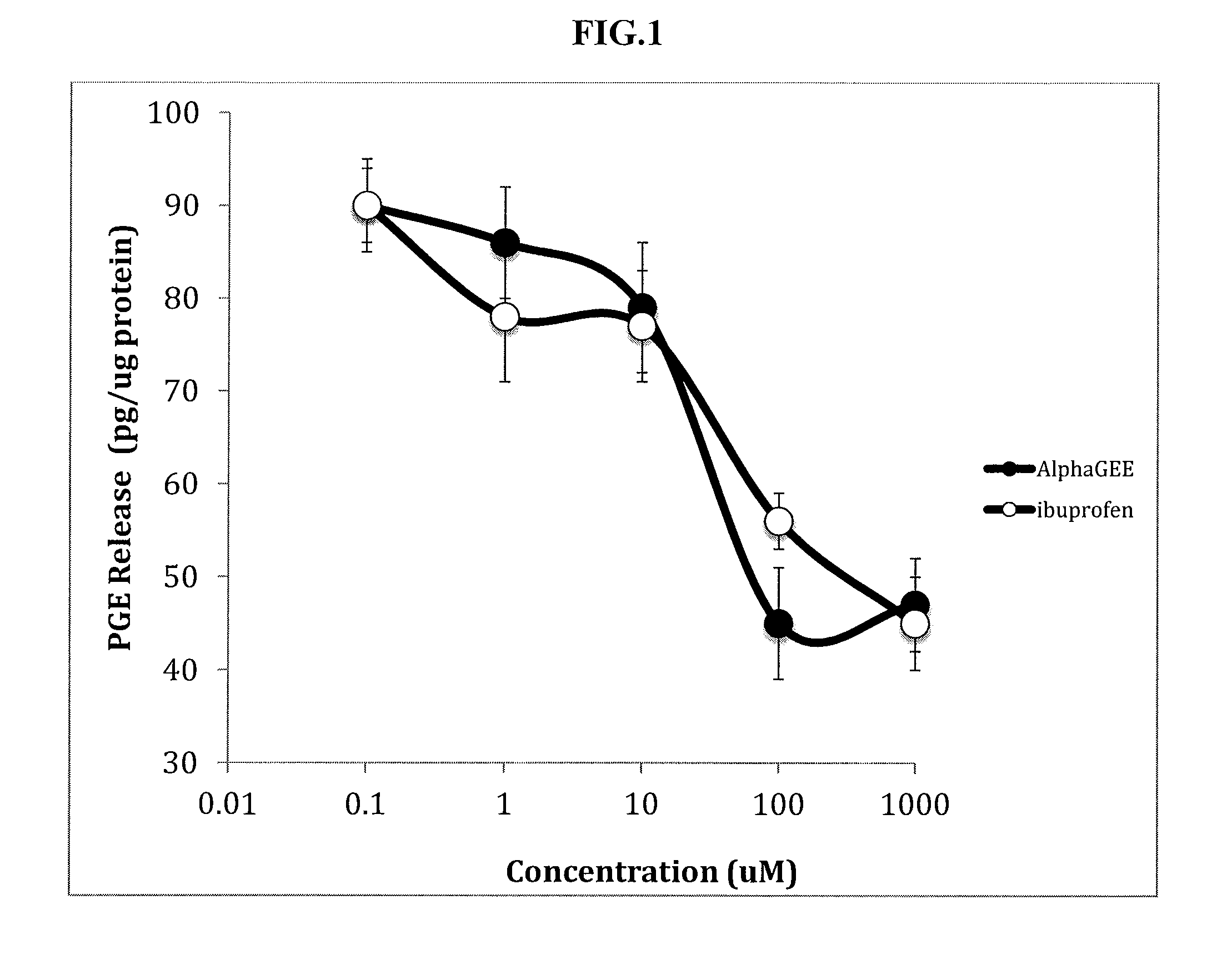
[0052]In this example, the effects of Alpha-GEE and Ibuprofen on the release of PGE2 from brain endothelial cells were compared. The brain endothelial cells were first exposed to bacterial endotoxin. The effects of Alpha-GEE and Ibuprofen were then measured and the levels of release of PGE2 were compared.
[0053]FIG. 1 demonstrates that the reduction in the release of PGE2 from brain endothelial cells at an Alpha-GEE concentration of about 20 μM or more is about 50 percent or more. The reduction in the release of PGE2 from brain endothelial cells at an Alpha-GEE concentration of about 1000 μM or more greater than about 50 percent. The reduction of PGE2 release observed with Alpha-GEE is similar in magnitude to the reduction of PGE2 release observed with ibuprofen treatment,
example 3
Effects of Alpha-GEE and Ibuprofen on Inhibition of Cyclooxygenase (“COX”) Activity
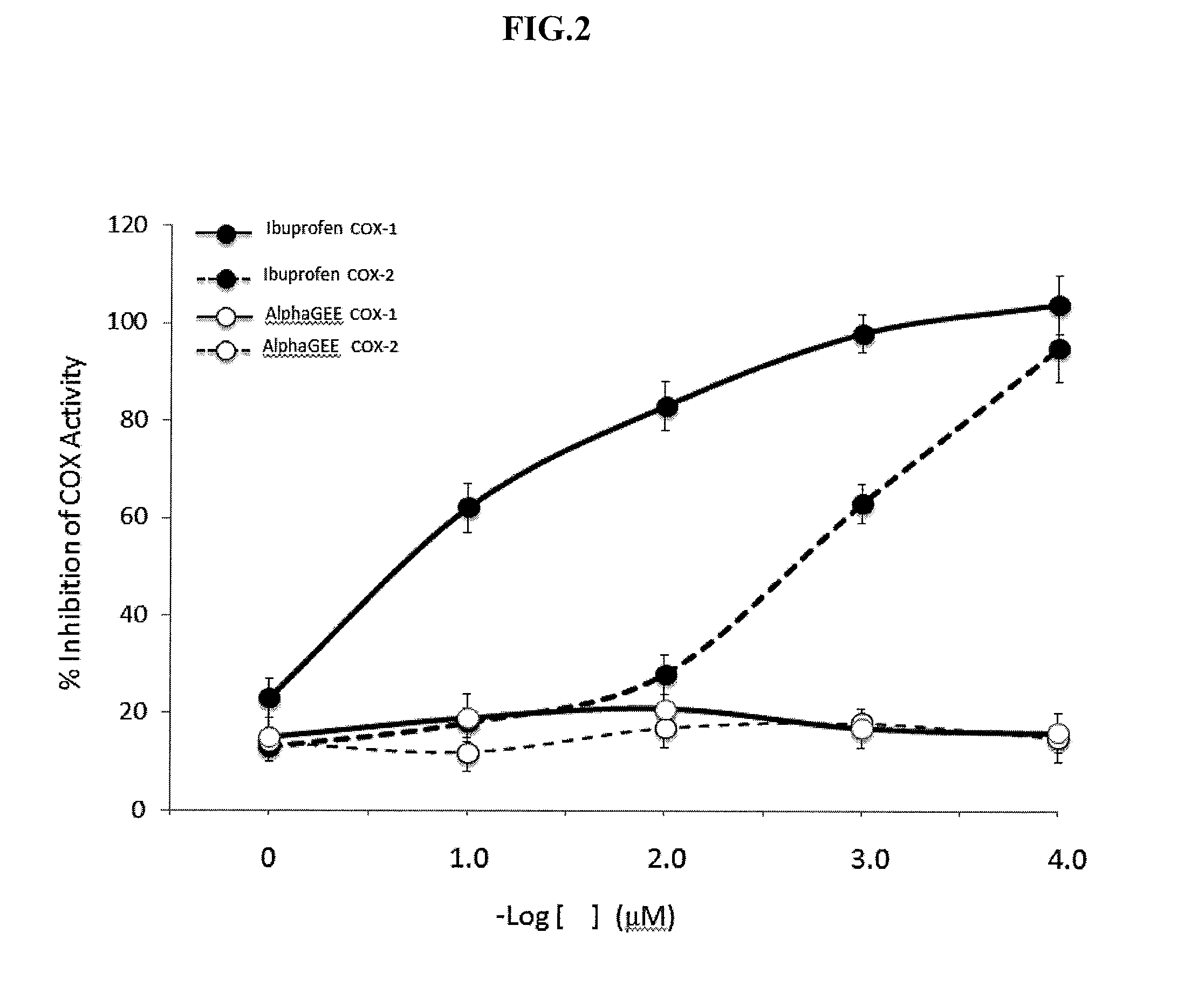
[0054]The effects of Alpha-GEE and Ibuprofen on the inhibition of cyclooxygenase-1 (“COX1”) and cyclooxygenase-2 (“COX2”) activity were also compared. As shown in FIG. 2, NSAIDs, such as ibuprofen, chemically bind to cyclooxygenase and inhibit prostaglandin production. Specifically, the NSAID, Ibuprofen, produced a concentration dependent inhibition of the COX enzymes when used at concentrations of 0.1-100 μM. For example, greater than about 60 percent of COX-1 and COX-2 activity is inhibited with Ibuprofen. In contrast, Alpha-GEE did not show a concentration dependency on the inhibition of COX enzymes. In other words, Alpha-GEE did not show a mechanistic inhibition of the COX enzymes. This suggests that the anti-inflammatory pathway for Alpha-GEE is different from that of NSAIDs commonly used to treat conditions such as OA.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com