Identification of patients with abnormal fractional shortening
a fractional shortening and patient technology, applied in the field of identification of patients with abnormal fractional shortening, can solve the problems of limited cost-benefit consideration for echocardiographic screening for abnormal mfs, often not tested or overlooked for abnormal midwall fractional shortening, and specialized imaging techniques. to achieve the effect of increasing risk
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Patient Cohort
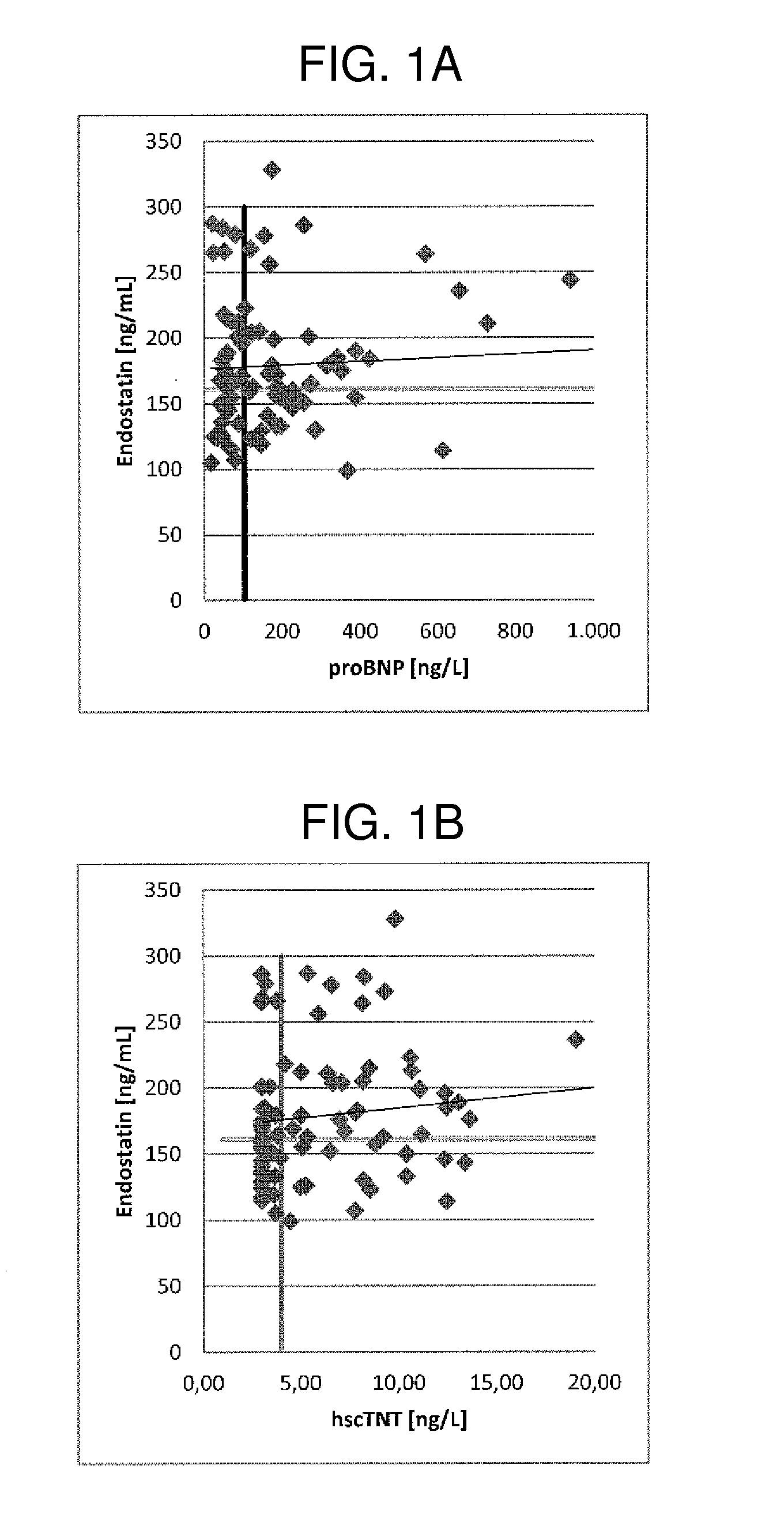
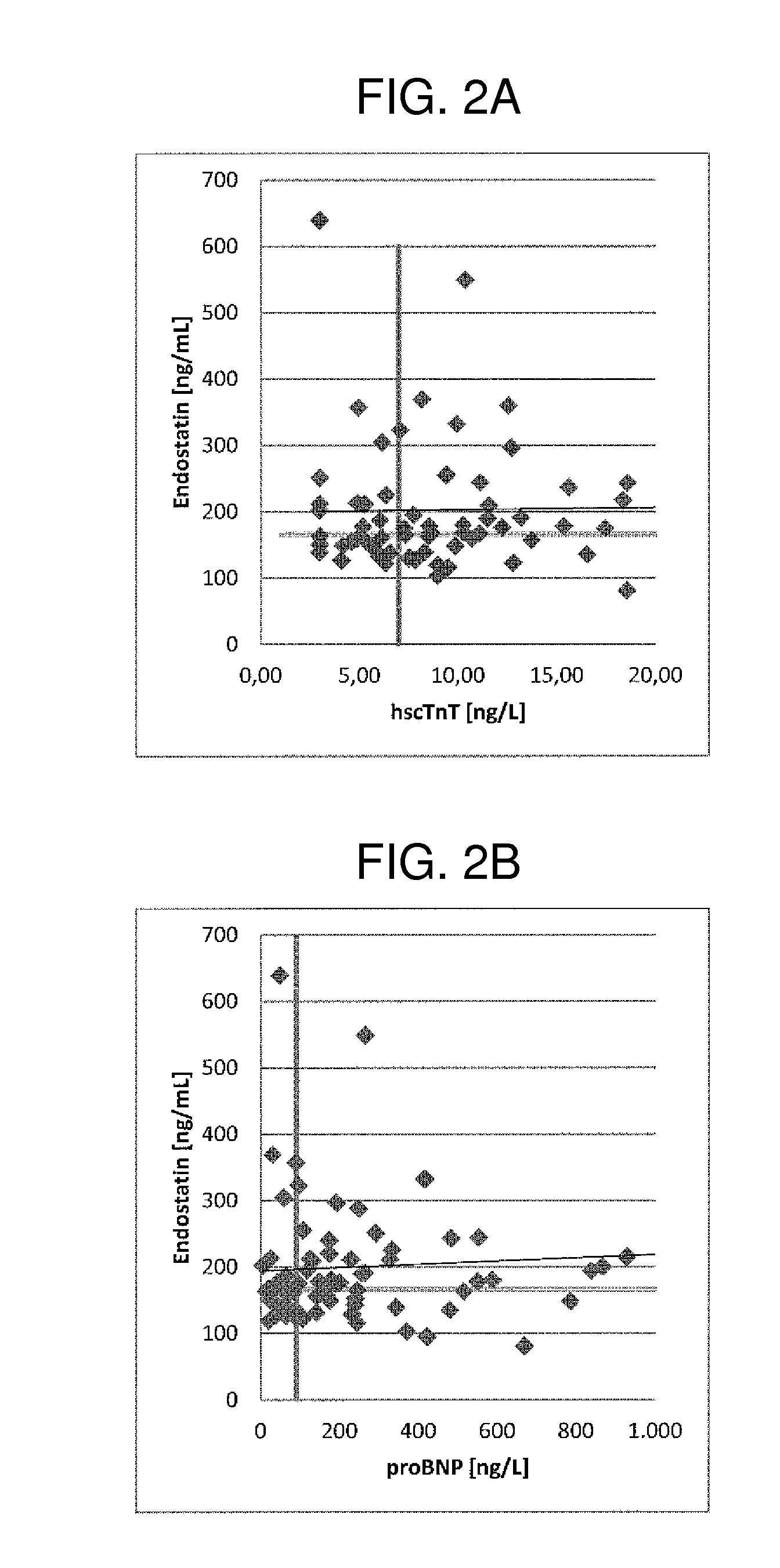
[0372]The relationship between plasma levels of FGF-23 (ELISA, Immutopics), total 25-hydroxyvitamin D (ECLIA, Roche Diagnostics), IFGBP7, endostatin, mimecan and 2 cardiac markers (hs-cTnT and NT-proBNP, Roche Elecsys assays) and elevated LV mass / BSA (>95 g / m2 for women, >115 for men), subnormal midwall fractional shortening (MFS<15%) and mortality was examined in 2001 elderly people (mean age 73±5 years, 48% women), resident in central Italy (the PREDICTOR study). The subjects were divided into 4 categories according to normal values of LV mass index and MF. The markers were determined in plasma samples. Data on all-cause mortality were available for a subgroup of 1200 subjects after a median follow-up of 47 months (86 deaths).
[0373]Three markers (Endostatin, IGFBP7 Mimecan / Osteoglycin) were examined in 550 elderly subjects selected from the 2001 subjects aged 65-84 years of the epidemiological study PREDICTOR. This subcohort comprises 50 normal subjects, 150 subject ...
example 2
Assays
[0375]Troponin T was determined using Roche's electrochemiluminescence ELISA sandwich test Elecsys Troponin T hs (high sensitive) STAT (Short Turn Around Time) assay. The test employs two monoclonal antibodies specifically directed against human cardiac troponin T. The antibodies recognize two epitopes (amino acid position 125-131 and 136-147) located in the central part of the cardiac troponin T protein, which consists of 288 amino acids. The hs-TnT assay allows a measurement of troponin T levels in the range of 3 to 10000 pg / mL.
[0376]NT-proBNP was determined using Roche's electrochemiluminescence ELISA sandwich test Elecsys proBNP II STAT (Short Turn Around Time) assay. The test employs two monoclonal antibodies which recognize epitopes located in the N-terminal part (1-76) of proBNP (1-108).
[0377]Human FGF-23 was determined by used the (C-Term) ELISA Kit from Immutopics Inc., Cat. Number 60-6100, 2nd Generation Enzyme-Linked ImmunoSorbent Assay (ELISA) for the Determination...
example 3
Results
Subtle Alterations of LV Geometry—Midwall Fractional Shortening
[0381]The results are shown in table 1. Median concentrations of hs-cTnT, NT-proBNP, FGF-23 and vitamin D were on average low. Median concentrations of hs-cTnT, Nt-proBNP and FGF-23 / Vitamin D were higher in subjects with elevated LV mass and / or or subnormal MFS (for both, adjusted for sex and age). FGF-23 and vitamin D were significantly associated to LV mass independently of hs-cTnT.
TABLE 1Categories of LV mass index / MFSCategories of LV mass index / MFSNormal LVMINormal LVMIElevated LVMIElevated LVMINormal MFSAbnormal MFSNormal MFSAbnormal MFSP*N 918 (57.8) 275 (17.3) 189 (11.9)206 (13) Age (year)72.1 (4.8) 73.3 (5.2) 72.9 (4.9) 74.3 (5.1) Females 444 (48.4) 108 (39.3) 130 (68.8) 120 (58.3)0.0525LV81.1 (14.1)88.0 (13.8)116.0 (18.0) 125.7 (22.8) mass / BSA(g / m2)MFS (%)17.4 (1.6) 13.5 (1.2) 16.5 (1.3) 12.8 (2.0) PMarkersNT-proBNP7880124182Hs-cTnT4.35.95.488.5Vitamin D13.5313.719.8810.640.0021[ng / ml]FGF-2369.174.076.284...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com