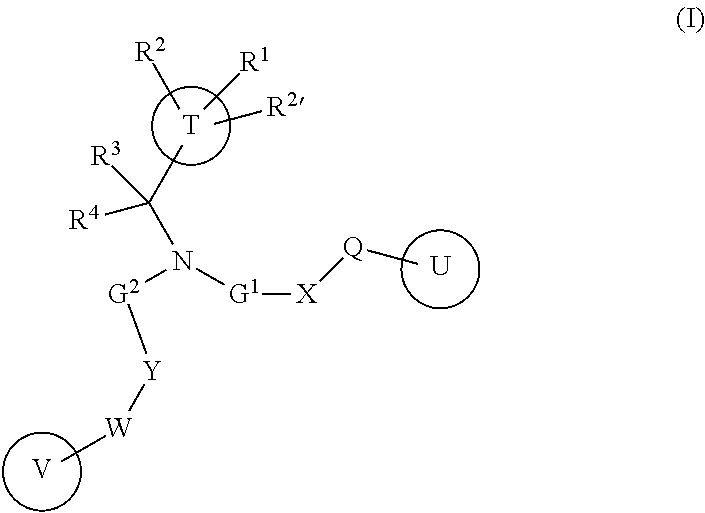
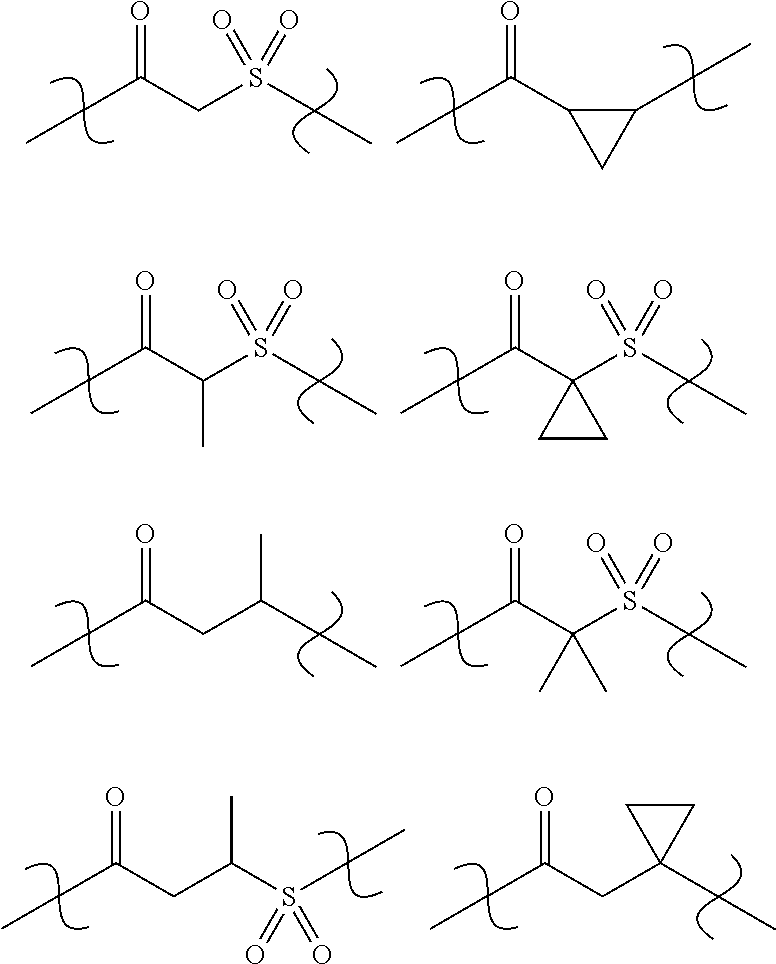
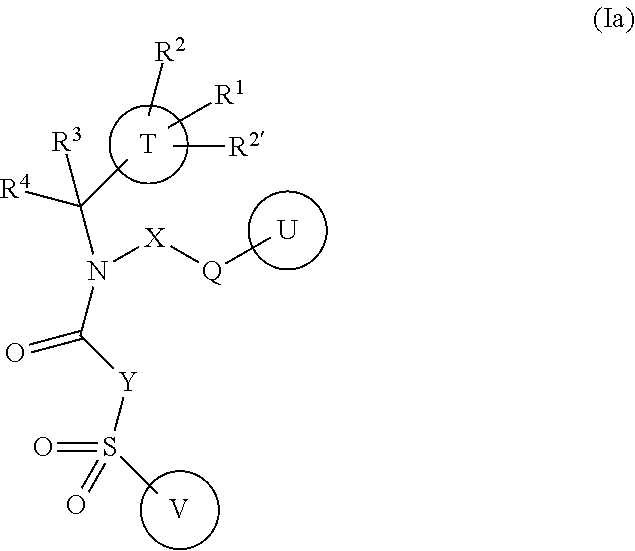
Amine Derivatives as Potassium Channel Blockers
a potassium channel and derivative technology, applied in the field of compounds useful in the modulation of the potassium channel activity in cells, can solve the problems of immune synapse formation, blockade of the channel, high blood sugar and other metabolic abnormalities,
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
N-[cyclopropyl(4-methoxyphenyl)methyl]-3-(4-fluorophenyl)-N-(pyridin-2-ylmethyl)butanamide
[0494]
[0495]Intermediate a (25 mg, 0.09 mmol) was reacted with Intermediate nn (46.7 mg, 0.23 mmol) in presence of triethylamine (26 μl, 0.19 mmol) according to General Procedure C to afford the title compound (34 mg, 84%) as clear oil. 1H NMR (CDCl3) δ 8.52-8.37 (m, 1H), 7.65-7.43 (m, 1H), 7.30-6.70 (m, 10H), 5.19-4.96 (m, 1H), 4.55-4.12 (m, 2H), 3.81-3.77 (m, 3H), 3.57-3.47 (m, 1H), 2.79-2.35 (m, 2H), 1.33-1.23 (m, 4H), 1.20-0.15 (m, 4H). 1H NMR (d6-DMSO, 110° C.) δ 8.42 (br s, 1H), 7.61-7.56 (m, 1H), 7.27-6.99 (m, 8H), 6.84-6.81 (m, 2H), 4.72-4.67 (m, 2H), 4.34 (t, J=17.7 Hz, 1H), 3.75 (s, 3H), 3.42-3.32 (m, 1H), 2.80-2.43 (m, 2H), 1.28-1.12 (m, 4H), 0.68-0.61 (m, 1H), 0.32-0.12 (m, 3H). LCMS (Method 2) Rt 2.346 min (98.2% purity), m / z 433.3 (M+H)+.
example 2
N-[cyclopropyl(4-methoxyphenyl)methyl]-3-(4-fluorophenyl)-N-(pyridin-2-ylmethyl)propanamide
[0496]
[0497]Intermediate a (30 mg, 0.11 mmol) was reacted with 3-(4-fluorophenyl)propanoyl chloride (41.7 mg, 0.22 mmol) in presence of triethylamine (31.2 μl, 0.22 mmol) according to General Procedure C to afford the title compound (32 mg, 68%) as clear oil. 1H NMR (CDCl3) δ 8.49-8.41 (m, 1H), 7.56-7.50 (m, 1H), 7.26-7.05 (m, 6H), 6.99-6.89 (m, 2H), 6.84-6.78 (m, 2H), 5.20-5.11 (m, 1H), 4.51-4.09 (m, 2H), 3.79 (br s, 3H), 3.06-2.90 (m, 2H), 2.75-2.47 (m, 2H), 1.04-0.95 (m, 1H), 0.79-0.66 (m, 1H), 0.39-0.28 (m, 2H), 0.12-0.04 (m, 1H). LCMS (Method 2) Rt 2.192 min (96.6% purity), m / z 419.2 (M+H)+.
example 3
(3R)—N-[cyclopropyl(4-chlorophenyl)methyl]-3-(4-fluorophenyl)-N-(pyridin-2-ylmethyl)butanamide
[0498]
[0499]Intermediate b (30 mg, 0.11 mmol) was reacted with Intermediate ei (44 mg, 0.22 mmol) in presence of triethylamine (31 μl, 0.22 mmol) according to General Procedure C to afford the title compound (42 mg, 88%) as clear oil. 1H NMR (CDCl3) δ 8.53-8.36 (m, 1H), 7.65-7.44 (m, 1H), 7.33-6.82 (m, 10H) 5.21-4.96 (m, 1H), 4.57-4.06 (m, 2H), 3.58-3.42 (m, 1H), 2.74-2.28 (m, 2H), 1.33-1.24 (m, 4H), 0.98-0.82 (m, 1H), 0.76-0.56 (m, 1H), 0.40-0.18 (m, 2H). HPLC (Method 2) Rt 2.831 min (100% purity). MS (ES+) m / z 437.3 (M+H+).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com