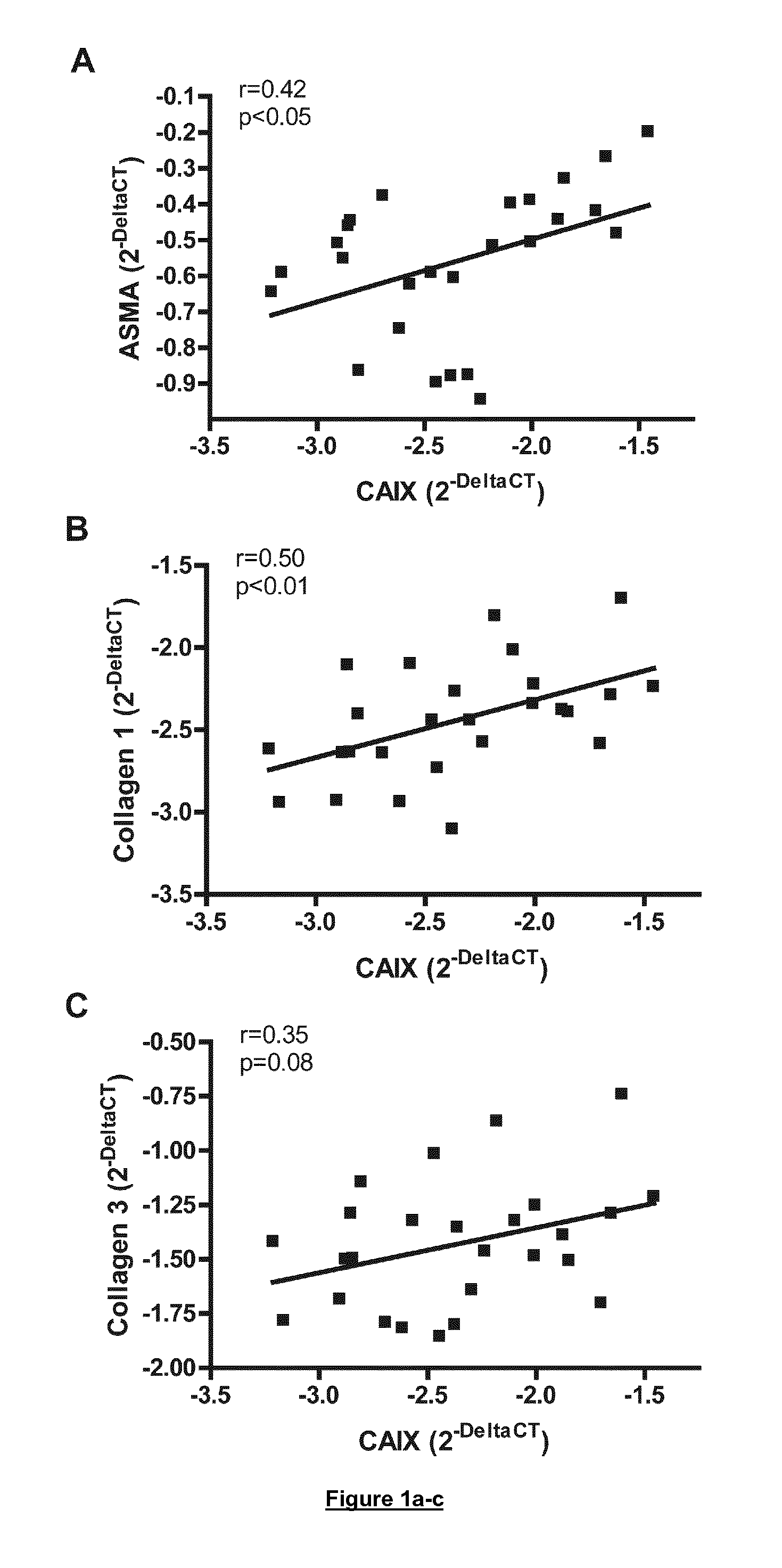
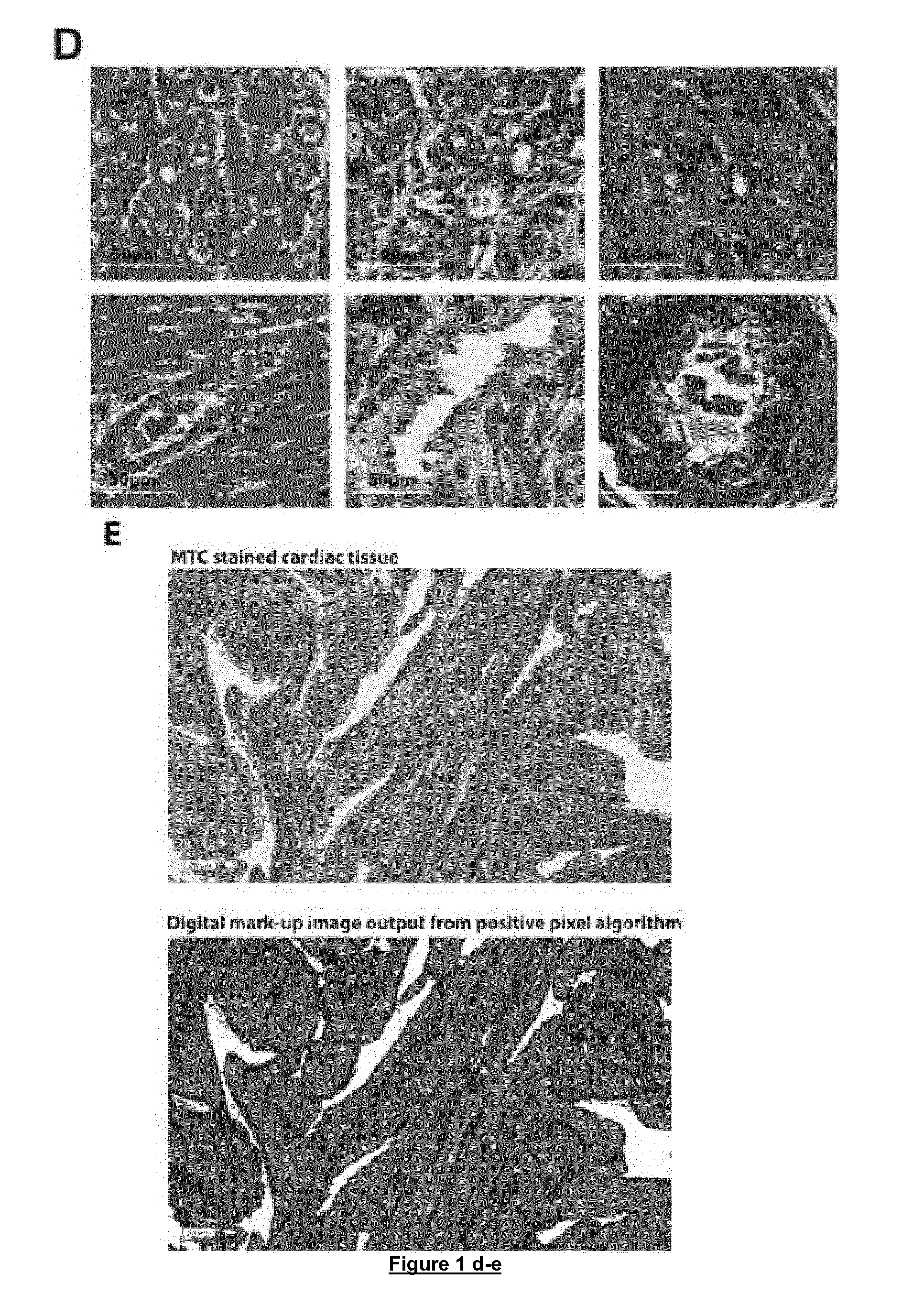
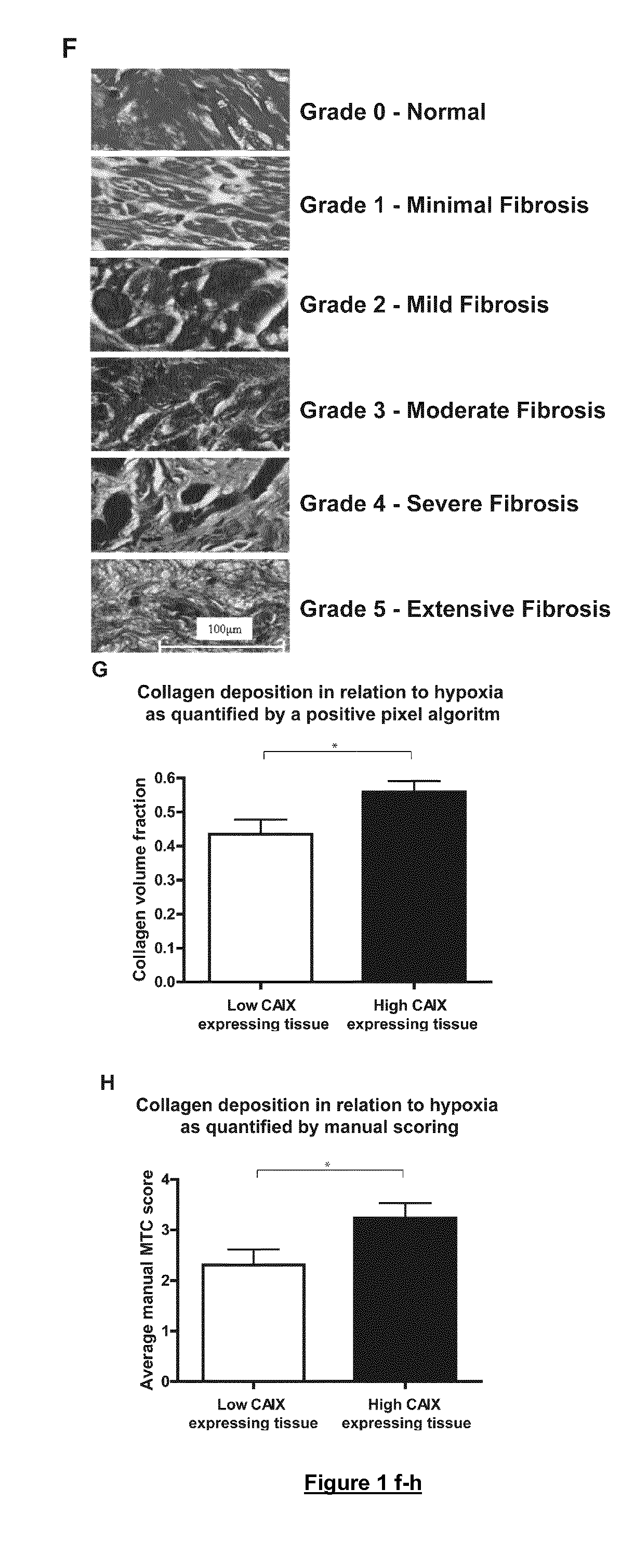
Therapies for cardiomyopathy
a cardiomyopathy and therapy technology, applied in the field of therapies and therapeutic agents, can solve the problems of difficult to place every cardiomyopathy in a discrete class, difficult to achieve the effect of reducing hypertrophy and fibrosis, and general poor
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 2
Epigenetic Therapy for the Treatment of Cardiac Fibrosis and Hypertrophy
[0128]Methods
[0129]Primary Cell Culture and Treatments
[0130]Primary human cardiac fibroblast cells from the adult ventricle (HCF) were purchased from ScienCell Research Laboratories. Cells were cultured and maintained in Dulbecco's modified eagles medium (DMEM) (Gibco), supplemented with 10% Fetal Bovine Serum (Gibco) and penicillin-streptomycin antibiotics (Gibco) in a 5% CO2 humidified incubator kept at 37° C. When required, HCF cells were treated for up to 8 days with either 10 ng / ml human recombinant transforming growth factor beta 1 (TGFβ1) (R&D Systems), 1 μM 5-azacytidine(5-aza) (Sigma), or with both compounds simultaneously.
[0131]Quantitative Real-Time PCR
[0132]RNA isolation from HCF cells was achieved using NucleoSpin RNA II Kit (Macherey-Nagel). First strand cDNA synthesis was carried out using SuperScript II RT (Invitrogen). Quantitative real-time PCR (QPCR) primers were designed so that one of each p...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
| thick | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com