Method for highly efficient conversion of human stem cells to lineage-specific neurons
a human stem cell and lineage-specific technology, applied in the field of stem cells, can solve the problems of lack of specificity and efficiency of strategies, and achieve the effects of high efficiency of human scs conversion, rapid and highly efficient lineage-specific neuronal conversion, and high application valu
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Proneural Transcription Factor Atoh1 Drives Highly Efficient Differentiation of Human Pluripotent Stems Cells into Dopaminergic Neurons
Materials and Methods
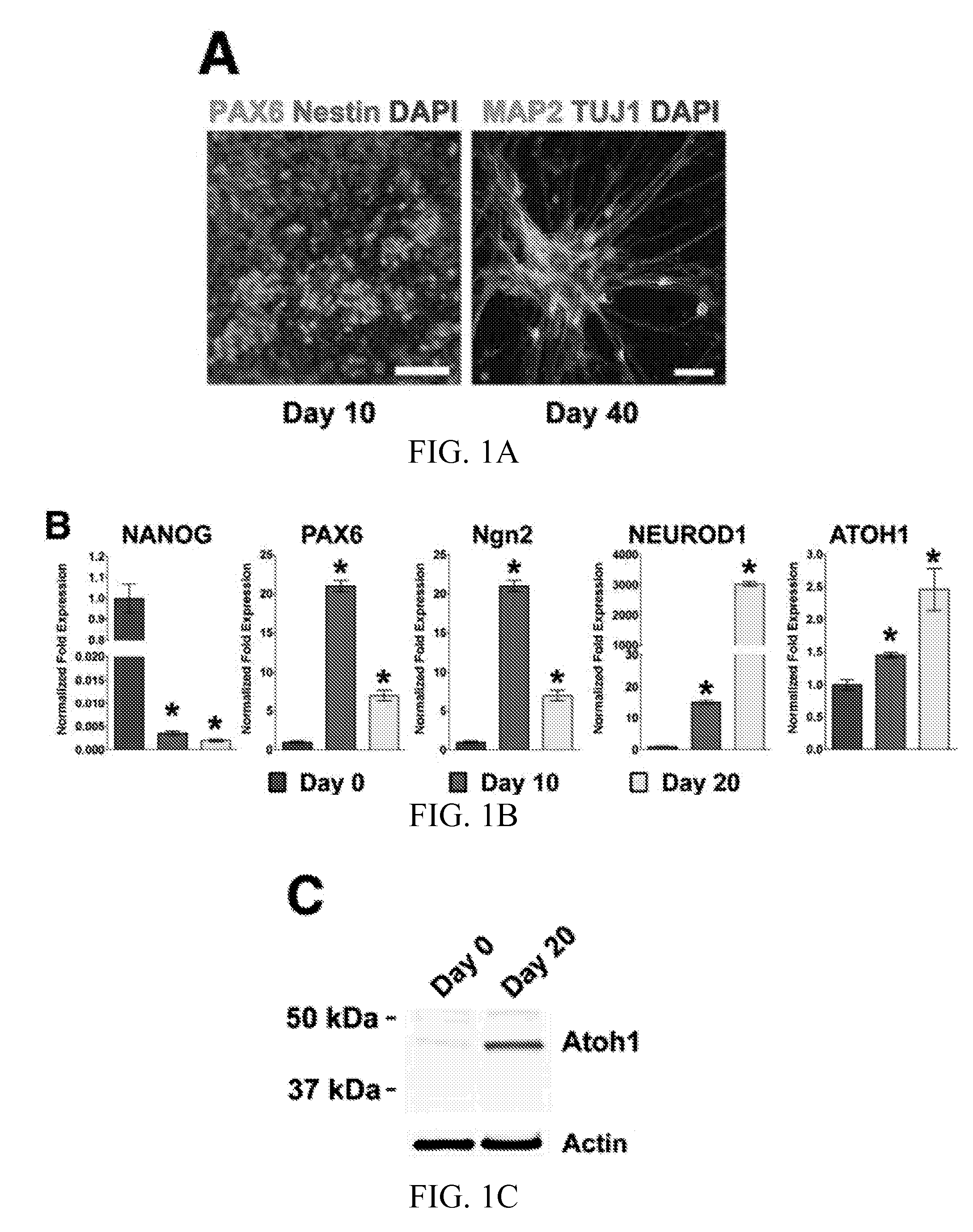
[0041]Cell Culture.
[0042]Human H1 ESC line was obtained from WiCell Research Resources (WiCell, WI). Human iPSC line ND27760 (passage 25-30) was derived from human skin fibroblasts from a PD patient with a SNCA triplication that were obtained from the Coriell Cell Repositories. Cell reprogramming was performed using non-integrating 4 factor (SOX2 / OCT4 / KLF4 / MYC) Sendai virus system (CytoTune-iPS Reprogramming Kit, Life Technologies). The pluripotency of this iPSC line has been characterized by immunocytochemistry for pluripotent cell markers (NANOG, OCT4, TRA-1-60 and SSEA-3) and embryoid body differentiation. Human ESCs and iPSCs were maintained as feeder-free cultures in Essential 8 medium (Life Technologies) or mTESR1 medium (Stemcell Technologies) in 5% CO2 / 95% air condition at 37° C., and were passaged using dispase (Life Tec...
example 2
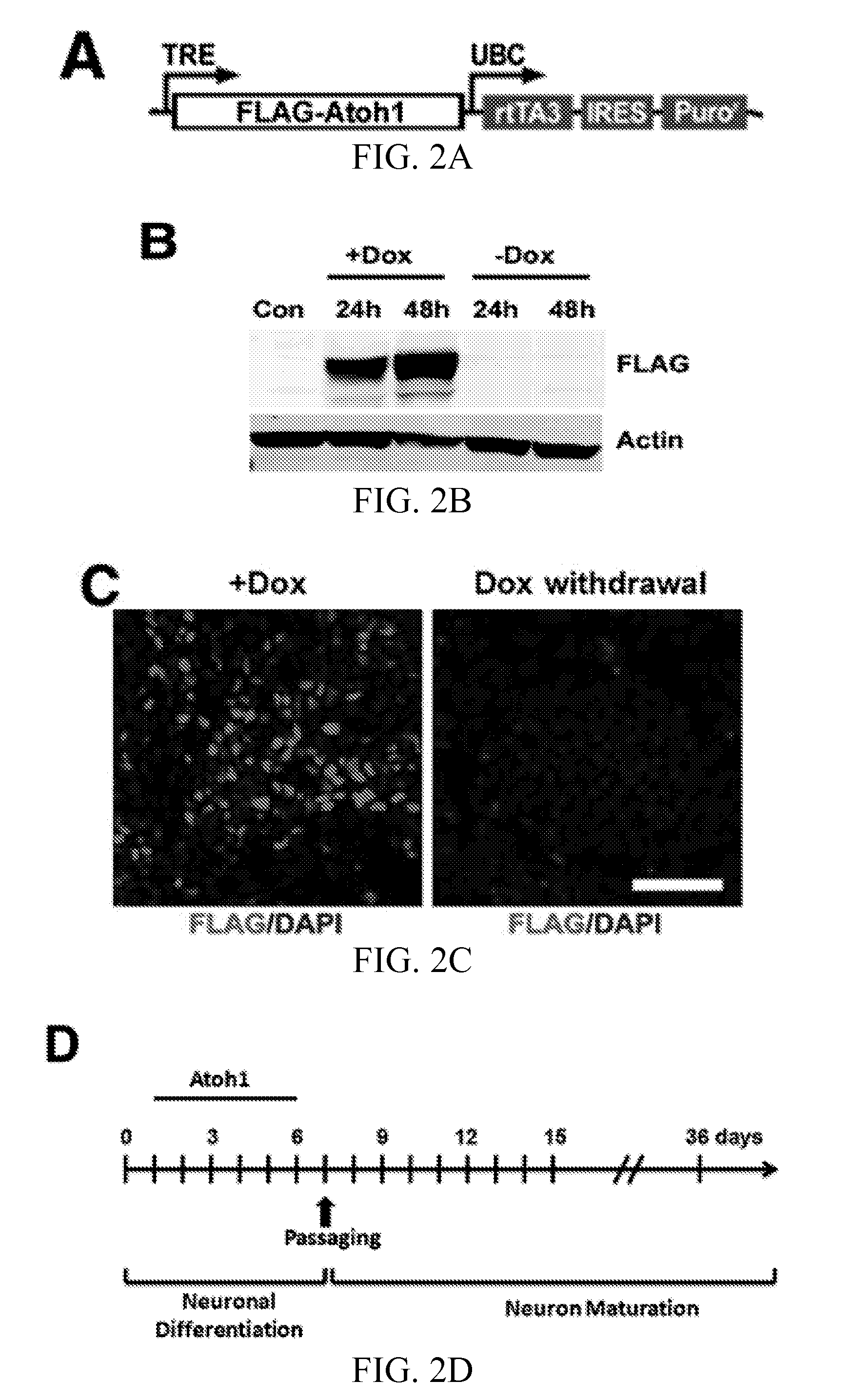
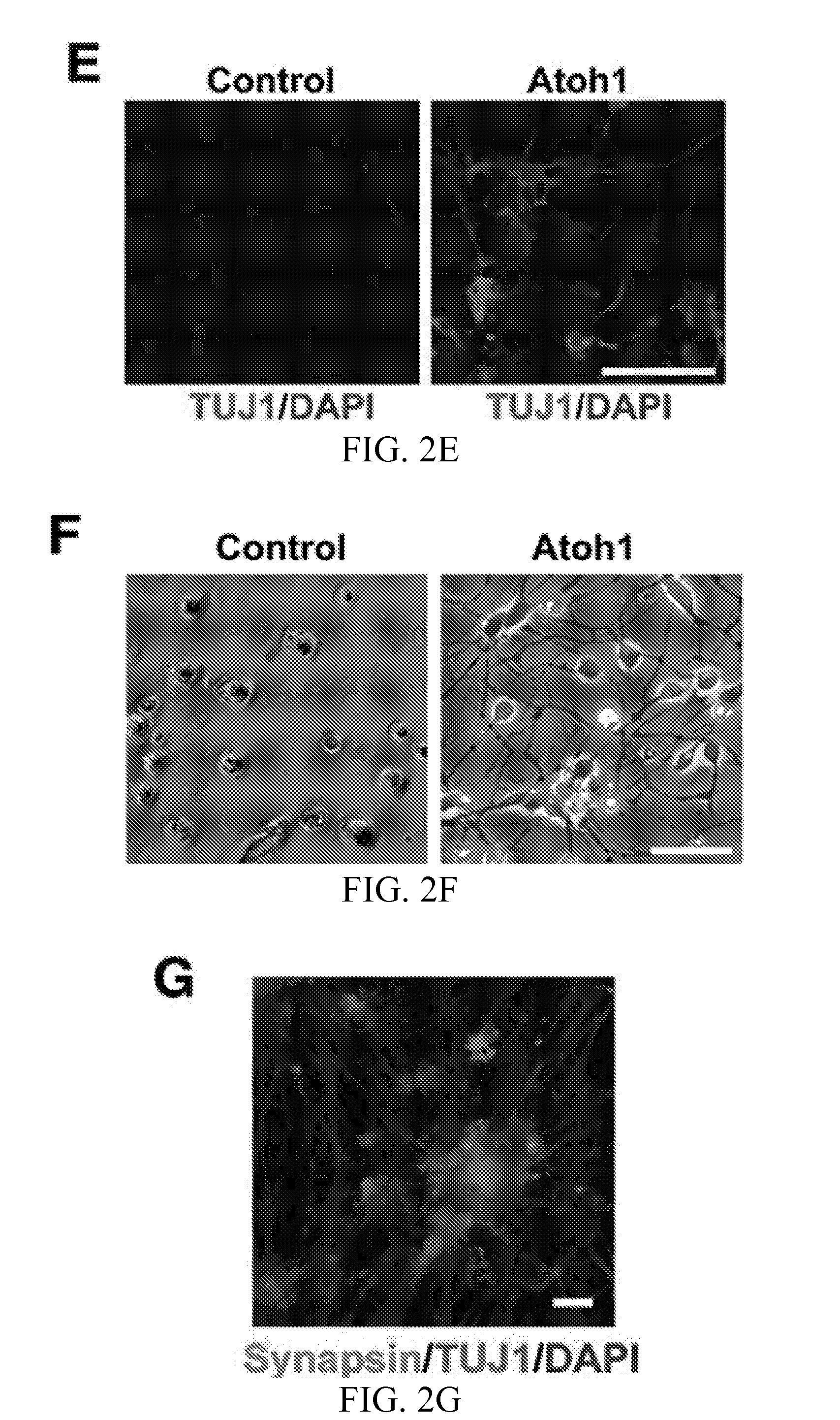
Atoh1-Mediated Differentiation of Human ESCs and NSCs into DA Neurons
[0092]Atoh1-ESCs were differentiated following the protocol as shown in FIG. 4A and Table 2. Atoh1-induced neuron cultures at Day 36 co-expressed neuronal and DA neuron markers, β-tubulin III (TUJ1) and tyrosine hydroxylase (TH), respectively (FIG. 12A). The Atoh1 induction protocol yielded DA neurons from human ESCs with 84±9% purity as determined by the percentage of TUJ+ / TH+ cells (FIG. 12A). Atoh1-NSCs were differentiated following the protocol as shown in FIG. 4A and Table 3. Atoh1-induced neuron cultures at Day 36 co-expressed neuronal marker (β-tubulin III, TUJ1) and DA neuron marker (tyrosine hydroxylase, TH) (FIG. 12B). The Atoh1 induction protocol yielded DA neurons from human NSCs with 82±8% purity as determined by the percentage of TUJ+ / TH+ cells (FIG. 12B).
TABLE 2DA neuron differentiation media used in Atoh1-induced protocol as shown in FIG. 4ADayMediumAdditives0E8Y-276321E6 (75%) + N2 (25%)Doxycyline / ...
example 3
Atoh1-Mediated Differentiation of Human iPSCs into Dopaminergic (DA) Neurons
[0093]As shown in FIG. 4A, we established protocols for differentiating human iPSCs into DA neurons by Atoh1 and other additives (sonic hedgehog and FGF-8b). The recipes for cell culture media are listed in Table 2. Cells were plated (4×104 cells per cm2) on matrigel (BD) in Essential 8 Medium (Life Technologies) with ROCK inhibitor (Y-27632, Stemgent). From Day 1 to Day 7, cell culture medium was changed every day. From Day 8 to Day 36, half of the cell culture medium was changed every 3-4 days. At day 36 of differentiation, these neurons co-expressed neuronal marker (β-tubulin III, TUJ1) and DA neuron marker (tyrosine hydroxylase, TH), the rate-limiting enzyme in the synthesis of dopamine (FIG. 4C). The Atoh1 induction protocol yielded DA neurons with 89±6% purity as determined by the percentage of TUJ+ / TH+ cells (FIG. 4D). Moreover, the Atoh1-induced DA neurons also co-expressed other midbrain DA neuron m...
PUM
| Property | Measurement | Unit |
|---|---|---|
| time period | aaaaa | aaaaa |
| frequency | aaaaa | aaaaa |
| resting membrane potential | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com