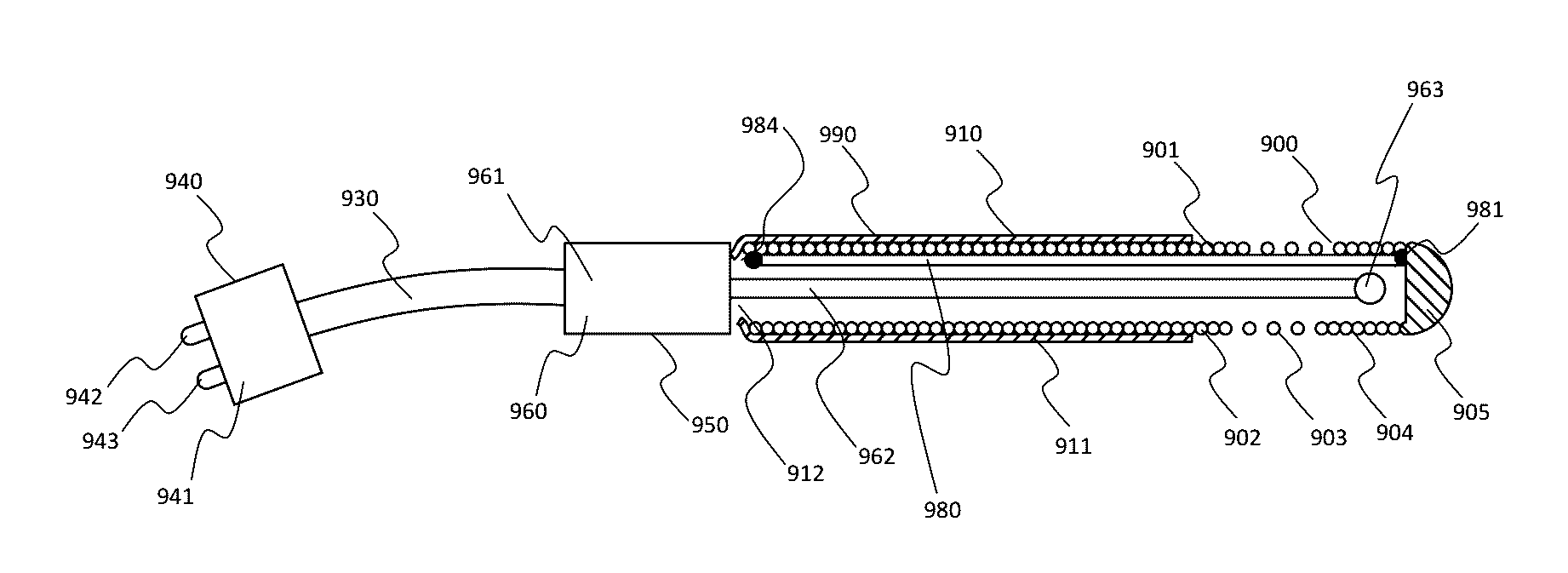
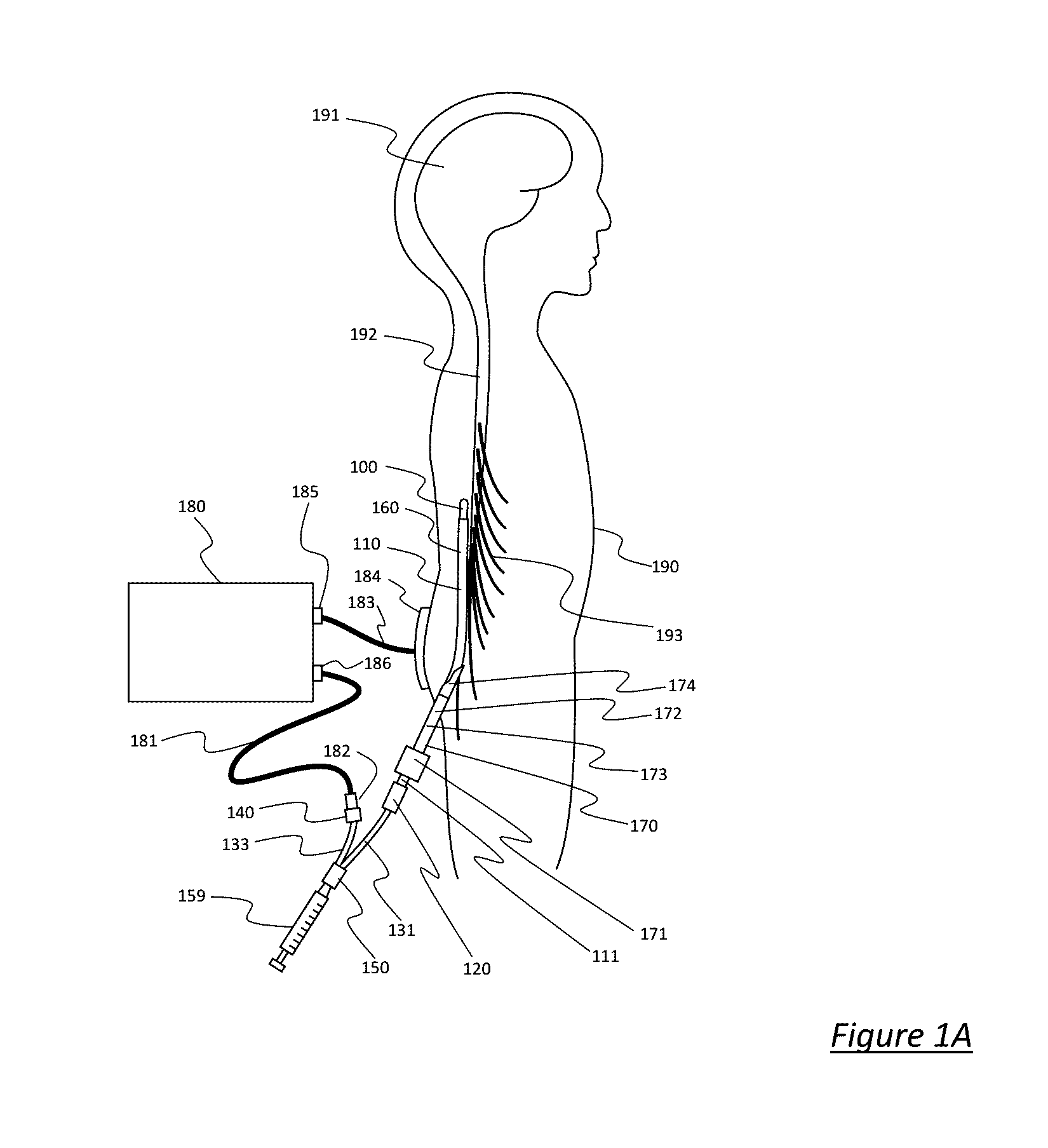
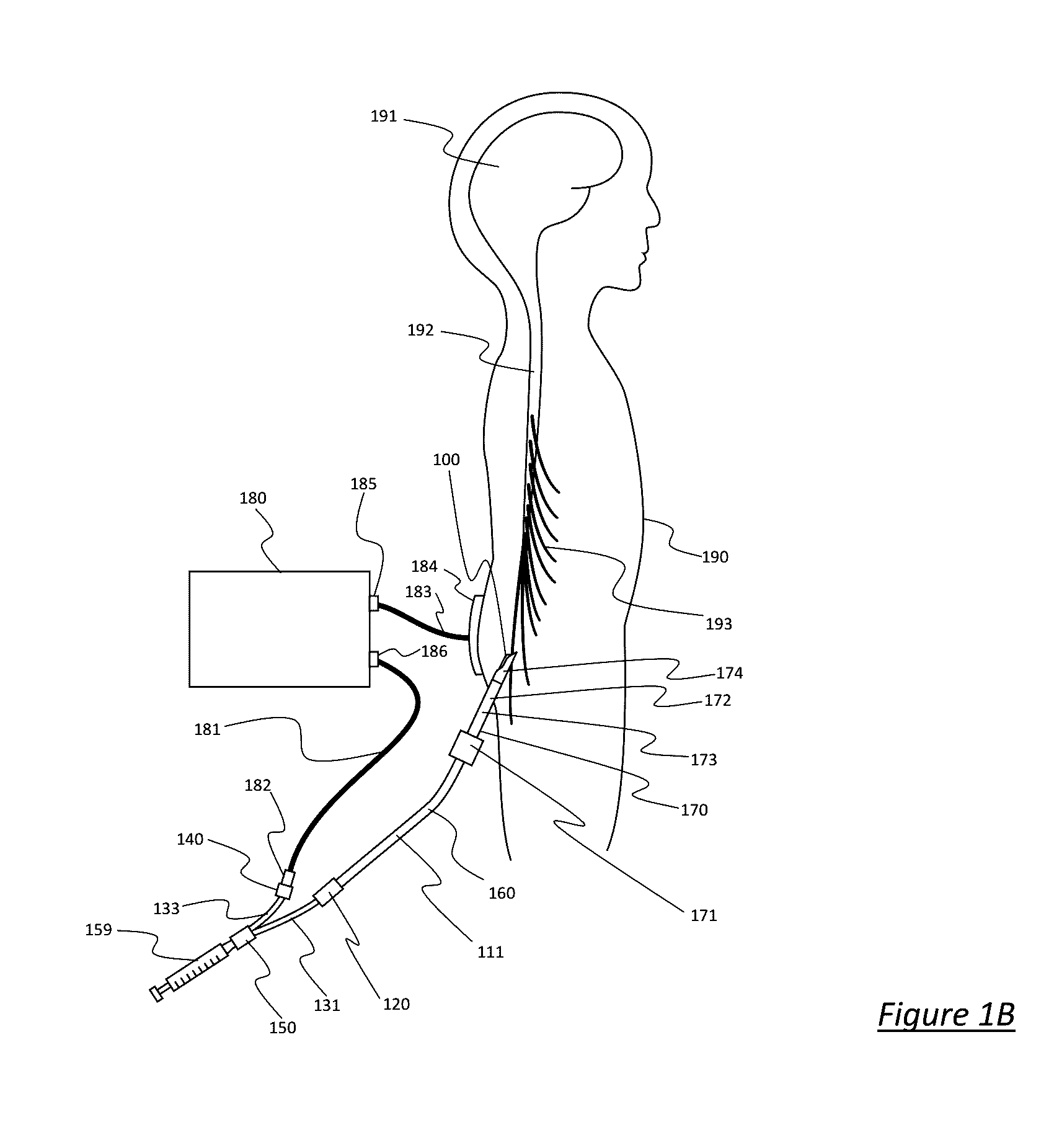
One limitation of such RF cannula and electrode systems, such as the Cosman CSK electrode and Cosman CC cannula, is that
fluid injection into the cannula cannot be achieved when the electrode is positioned within the cannula.
One limitation of such RF electrodes configured to be used with RF cannula is that they are not catheters.
One limitation of such RF electrodes configured to be used with RF cannula is that they are not configured for placement in the
epidural space.
One limitation of such RF cannulae is that they are not flexible enough to be guided through the
epidural space.
One limitation of such RF cannulae with sharp bevels is that they have sharp
cutting edges that can damage a
catheter that is introduced into a
living body though such a cannula.
One limitation of such RF cannulae with sharp bevels is that they can damage the dura and other sensitive structures if placed in the
epidural space.
One limitation of such RF cannulae in the prior art is that their bevels are not configured both to reduce the likelihood of damage to the dura and to reduce damage a catheter when a catheter is introduced into the epidural space through an RF cannula.
One limitation of such RF cannulae with sharp bevels is that they are not configured for the introduction of medical catheters, such as epidural catheters and catheter-type electrodes, into the
human body.
One limitation of blunt-tip RF cannulae is that they are not configured to introduce a catheter into the
human body through their inner lumen.
One limitation of the prior art in U.S. Pat. No. 7,862,563 is that it does not show a unitized injection electrode for which the metallic tip and insulated shaft are constructed using a spring coil and a central stiffening wire.
One limitation of the prior art in U.S. Pat. No. 7,862,563 is that it does not show the application of a unitized injection electrode in the epidural space.
One limitation of the prior art in RF injection electrodes is that they are not configured to be guided into and through the epidural space.
One limitation of the prior art in RF injection electrodes is that their tips are sharp.
One limitation of the prior art in RF injection electrodes is that their shaft does not include a spring coil.
One limitation of the prior art in RF injection electrodes is that they are not introduced into the
human body via an introducer needle.
One limitation of the prior art in RF injection electrodes is that they are not configured to introduce a catheter into the human body.
One limitation of the TEW electrode is that it is not configured to be threaded though the epidural space.
One limitation of the TEW electrode is that it is not configured to be threaded through 12 inches to 34 inches of the epidural space.
One limitation of the TEW electrode is that it is not long enough to apply RF therapy to multiple spinal nerves via a single
skin puncture and the epidural space.
One limitation of the TEW electrode is that it does not have an integral
injection port.
One limitation of the TEW electrode is that it is that it not configured to allow for outflow of fluids from its spring coil tip.
One limitation of the TEW cannula is that it is configured for the introduction of a catheter into the human body.
One limitation of the TEW cannula is that it does not have an active tip.
One limitation of the TEW cannula is that when an electrode is placed within its inner lumen, electrical signals applied to the cannula by the electrode are not transmitted to tissue in contact with any substantial part of the cannula.
One limitation of the TEW cannula is that it does not have an tip characteristic of an epidural needle.
One limitation of the TEW cannula is that it is not configured for placement in the epidural space and to perform RF therapy in the epidural space.
One limitation of the Flextrode electrode is that it is not configured for placement in the epidural space.
One limitation of the Flextrode electrode is that it is configured for injection of fluids into the human body.
One limitation of the Flextrode cannula is that its bevel is not configured for the introduction of catheters into the human body.
One limitation of the Flextrode cannula is that its bevel is not configured for the introduction of catheters whose external surface is soft material, such a plastic, into the human body.
One limitation of the Flextrode cannula is that its bevel is not an epidural bevel, such as a tuohy bevel, RX bevel, Wavepoint bevel, Cath Glide bevel, or the bevel shown in Higuchi.
One limitation of the Flextrode cannula is that sharp surfaces of its bevel can damage a soft catheter passing through it.
One limitation of the DiscTrode electrode is that it is not configured for placement in the epidural space.
One limitation of the DiscTrode electrode is that it is configured for injection of fluids into the human body.
One limitation of the DiscTrode cannula is that its bevel is not configured for the introduction of catheters into the human body.
One limitation of the DiscTrode cannula is that its bevel is not configured for the introduction of catheters whose external surface is soft material, such a plastic, into the human body.
One limitation of the DiscTrode cannula is that its bevel is not an epidural bevel, such as a tuohy bevel, RX bevel, Wavepoint bevel, Cath Glide bevel, or the bevel shown in Higuchi.
One limitation of the DiscTrode cannula is that sharp surfaces of its bevel can damage a soft catheter passing through it.
One limitation of the spinecath catheter is that it is not configured for placement in the epidural space.
One limitation of the spinecath catheter is that it is not configured for injection of fluids into the human body.
One limitation of the spinecath catheter is that it is not a radiofrequency electrode with an active tip.
One limitation of the spinecath catheter is that it does not apply RF signals to tissue that are in contact with it.
One limitation of the spinecath cannula is that it is not configured to function as an RF cannula.
One limitation of the spinecath cannula is that it does not have an epidural bevel, such as a tuohy bevel, RX bevel, Wavepoint bevel, Cath Glide bevel, or the bevel shown in Higuchi.
One limitation of the spinecath cannula is that it is not configured for introduction of epidural catheters.
One limitation of the spinecath cannula is that it is not electrically insulated.
One limitation of the prior art in epidural catheters is that an electrode with a
temperature monitoring is not used as a stylet.
One limitation of the prior art in epidural catheters is that the stylet does not have an integrated connection cable to an electrical generator.
One limitation of the prior art in epidural catheters is that the stylet does not have an integrated connection cable to an RF generator that includes both a wire for conducting an RF signals and a wire for conducting temperature signals.
One limitation of the prior art in epidural catheters is that prior catheters do not provided for temperature-controlled RF lesioning.
One limitation of the prior art in epidural catheters is prior catheter systems are not a unitized injection electrode.
One limitation of the prior art in epidural catheters is prior catheter systems are not a unitized injection electrode whose shaft includes a spring coil.
One limitation of the prior art in introducer needles for catheters is that the needles are not electrically insulated along their shafts.
One limitation of the prior art in introducer needles for medical catheters is that the needles do not both provide a tip configured for introduction of a catheter, and provide an insulated shaft that defines an active tip for the targeted delivery of electrical signals, such as
nerve stimulation signals and RF signals.
One limitation of the prior art in introducer needles for catheters is that the needles' most distal bevel surface is not curved when viewed from the side of the bevel.
One limitation of the prior art in injection adaptors for epidural catheters is that fluid cannot be injected into the injection adaptor when the stylet is inserted into the injection adaptor.
One limitation of the prior art in injection adaptors for epidural catheters is that the injection adaptors do not include
two fluid seals.
One limitation of the prior art in injection adaptors for epidural catheters is that the adaptors do not include a first fluid seal for connection to a catheter and a second fluid seal to prevent outflow from the stylet port.
One limitation of the prior art in injection adaptors for epidural catheters is that the adaptors do not contain three ports, one for the catheter, one for the stylet, and one for injection of fluids.
One limitation in stylets for epidural catheters is that the stylet does not provide a port for injection into the catheter into which the stylet is placed.
One limitation of the needle in Higuchi is that it has a gentle curve at its distal end.
One limitation of the needle in Higuchi is that the shaft is not substantially straight at the bevel.
One limitation of the Wavepoint
epidural needles is that they are not electrically insulated.
One limitation of the art presented in U.S. Pat. No. 6,246,912 is that the
catheter electrode does not provide for the injection of fluids.
One limitation of the art presented in U.S. Pat. No. 6,246,912 is that the
catheter electrode does not apply
high frequency signals to the tissue by the same spring coil that is part of its shaft construction.
One limitation of the prior art in Betts is that the catheter has an adaptor hub.
One limitation of the
system described in Betts is that a standard epidural catheter is not used.
One limitation of the
system described in Betts is that construction of the catheter using a
metal coil is not described.
One limitation of the
system described in Betts is that a safety strap within the catheter shaft is not described.
One limitation of the absence of a metallic safety strap is that the impedance of the catheter shaft can distort and / or diminish electrical signals conducted along the shaft.
One limitation of the system described in Betts is that RF is not delivered without seating of the RF wire in the inner surface of the distal end of the catheter.
One limitation of the system described in Betts is that the system does not provide for
temperature monitoring.
One limitation of the system described in Betts is that the system does not provide for temperature-monitored RF therapy delivered through the catheter.
One limitation of the system described in Betts is that the RF wire does include a temperature sensor.
One limitation of the system described in Betts is that it is not a unitized injection electrode.
One limitation of the system described in Betts is that the RF wire is separate from the catheter.
One limitation of the system described in Betts is that injection through the catheter cannot be effected while the RF wire is in place within the catheter.
One limitation of the system described in Betts is that it does not provide for simultaneous injection of fluids and delivery of electrical signals.
One limitation of the prior art in Betts is that the needle is not covered by electrical insulation to define an active tip.
Another limitation of the prior art in Betts is that the needle is not connected to an electrical
energy source, such as a stimulator, or an RF generator.
Another limitation of the prior art in Betts is that the introducer needle for a medical
catheter electrode is not used as a path for return currents from the medical catheter electrode.
One limitation of the prior art in Betts is that fluid cannot be injected through the
catheter hub and into the catheter when the metallic wire element is telescopically disposed within a lumen of the catheter.
One limitation of the
catheter hub described in Betts is that it does not provide for simultaneous injection of fluids and delivery of electrical signals.
One limitation of the
catheter hub is does not describe more than one fluid clamp.
One limitation of the prior art in Omar-Pasha is that it does not describe the use of a coil to construct the catheter electrode.
Another limitation of the prior art in Omar-Pasha is it does not describe an RF electrode system in which an RF electrode stylet is inserted into a standard epidural catheter.
One limitation of the prior art in Omar-Pasha is that the catheter electrode does not have a stylet port, nor a stylet port with a clamp.
One limitation of the prior art in Omar-Pasha is that the catheter electrode does not have a separable injection adaptor.
One limitation of the Pulsetrode is that it does not describe the use of a coil to construct the catheter electrode.
One limitation of the Pulsetrode is that an
active electrode tip of the catheter is constructed from the same coil that is included in the catheter shaft.
Another limitation of the Pulsetrode is that it is not an RF electrode system in which an RF electrode stylet is inserted into a standard epidural catheter.
Another limitation of the Pulsetrode is that the distal end of the electrode is electrically insulated.
Another limitation of the Pulsetrode is that the active tip is not the sole active tip.
One limitation of the Pulsetrode electrode is that the stylet must be removed from the electrode in order that injection take place.
Another limitation of the pulsetrode electrode is that the stylet port is not capable of producing a fluid-tight seal around the stylet.
Another limitation of the pulsetrode electrode is that the
injection port is not separate from the stylet port.
Another limitation of the pulsetrode electrode is that the
injection port is not separable from the catheter.
The Cool-Tip electrode has the limitation that it does not provide a hollow tube for injection of fluids.
The Cool-Tip electrode has the limitation that it is straight.
 Login to View More
Login to View More  Login to View More
Login to View More