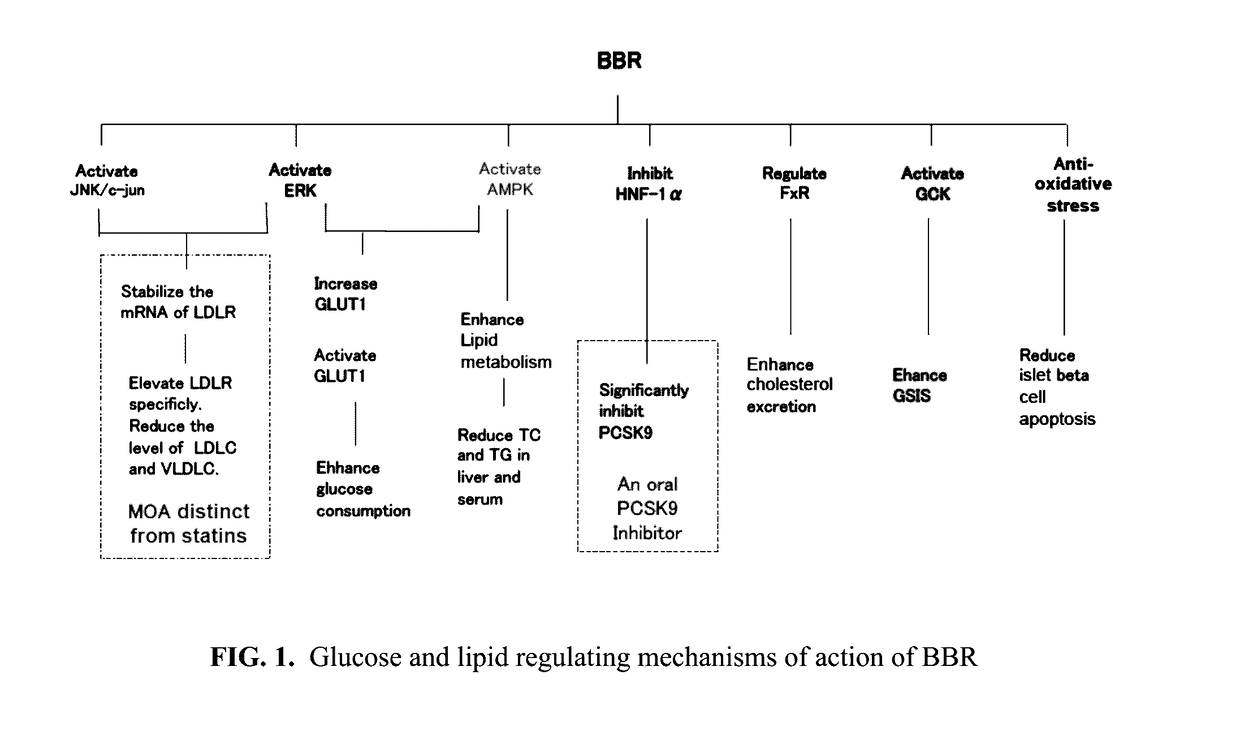
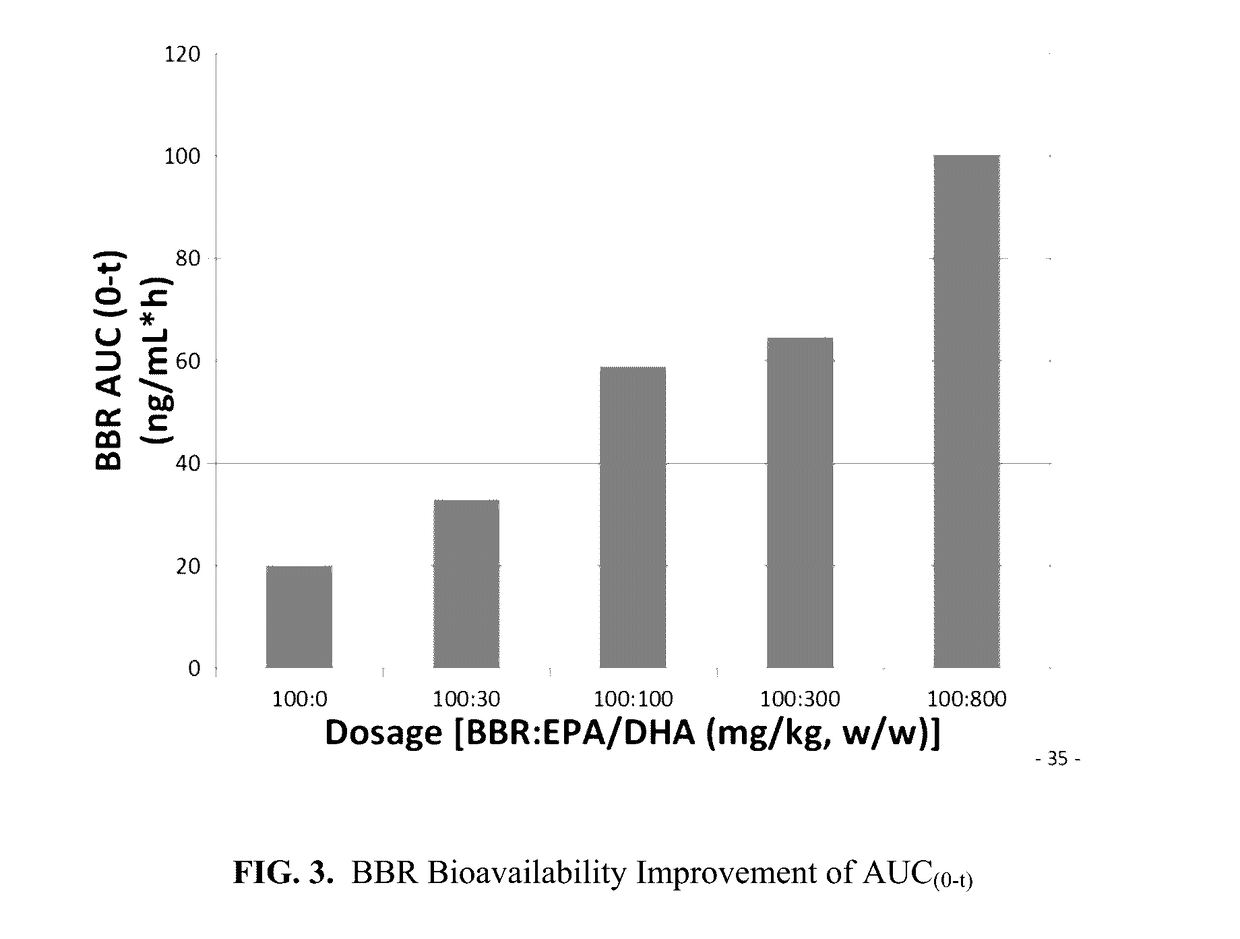
Pharmaceutical compositions of berberine with epa and dha, and methods thereof
a technology of berberine and epa, which is applied in the directions of medicine preparations, capsule delivery, metabolism disorders, etc., can solve the problems of hyperglycemia, heart attack and death, and potentially serious conditions such as hyperglycemia, and achieve the effect of treating, reducing, or preventing a disease or disorder
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Pharmacokinetic Properties of the Combination of Berberine and EPA / DHA in Rats and Dogs
[0091]This example describes the in vivo studies in rats and dogs on the pharmacokinetic (PK) properties of the combination of berberine (BBR) and EPA and / or DHA disclosed in the present invention.
[0092]In the single-dose pharmacokinetics study in rats, after 7-day acclimation, healthy male Sprague-Dawley (SD) rats with the body weight of 210-250 g were randomized into four groups as follows (3 rats per group).
GroupCompositionsVehicle Control0.5% gum tragacanthBerberine Hydrochloride BBR•HCl 51.13 mg / Kg(BBR•HCl)EPA Ethylester (EPA-EE)EPA-EE 45.48 mg / KgBBR + EPA-EEBBR•HCl 51.13 mg / Kg + EPA-EE 45.48 mg / Kg
[0093]The rats in each group were orally treated with the suspensions of the corresponding testing articles indicated above in 0.5% gum tragacanth respectively. Blood samples of the test animals were collected pre-dose and at the time points of 15 min, 30 min, 1 h, 1.5 h, 2 h, 4 h, 8 h, 24 h post-do...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Mass | aaaaa | aaaaa |
| Mass | aaaaa | aaaaa |
| Mass | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com