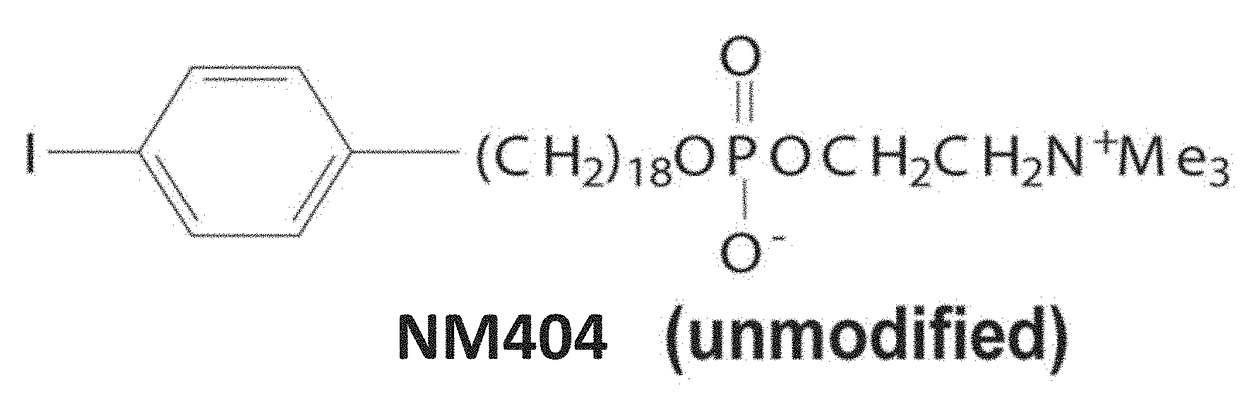
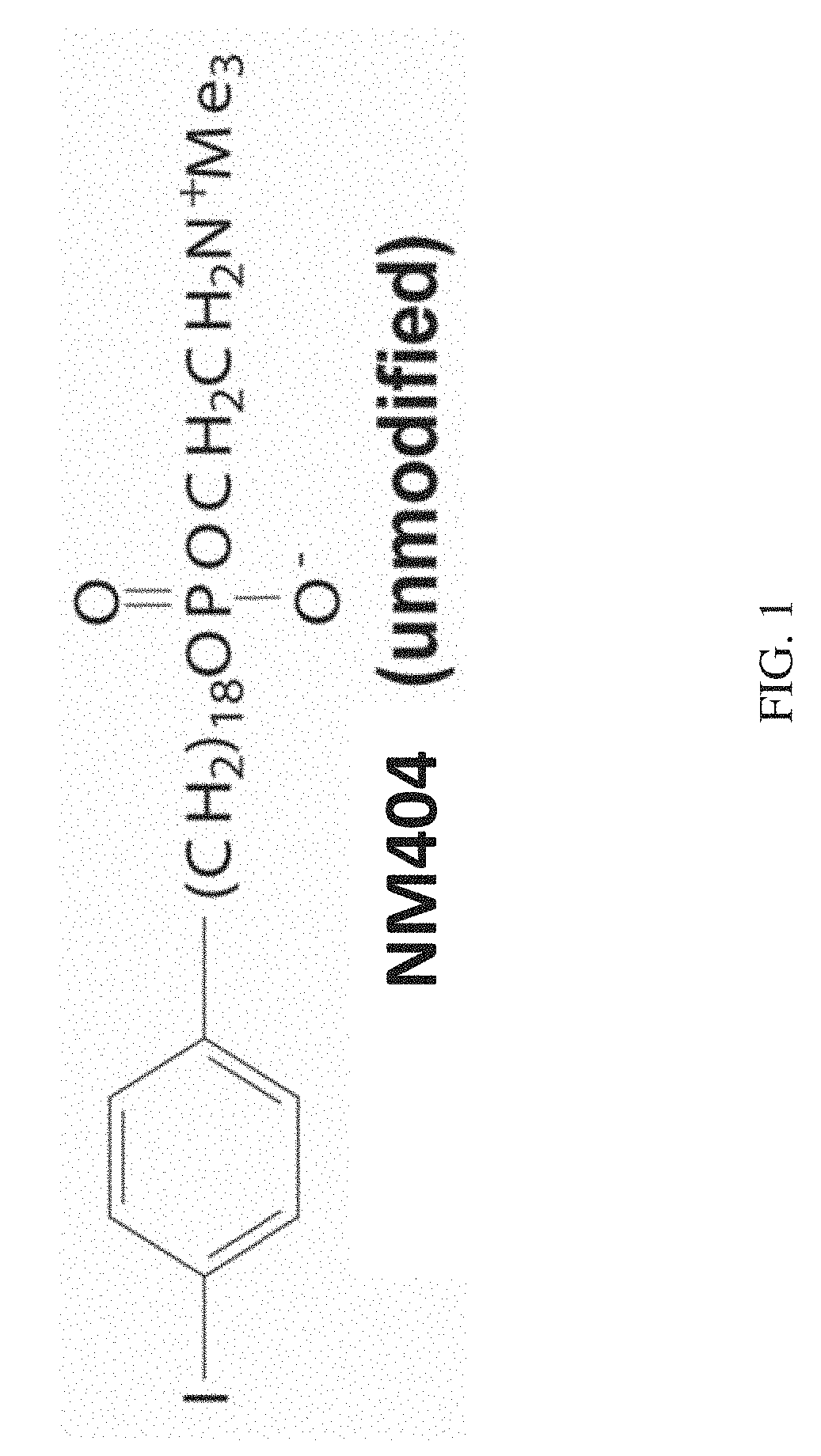
Radioactive phospholipid metal chelates for cancer imaging and therapy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
of Metal Chelated DO3A-404
[0119]In this Example, we show the synthetic scheme used to synthesize one exemplary phospholipid chelate, Gd-DO3A-404. Analogs incorporating various radioactive isotopes could be synthesized in a similar manner, where the radioactive isotope in questions is substituted for Gd.
[0120]Scheme for synthesizing Gd-DO3A-404 (the disclosed radioactive metal isotopes can be readily substituted for Gd):
example 2
maging Proof of Concept
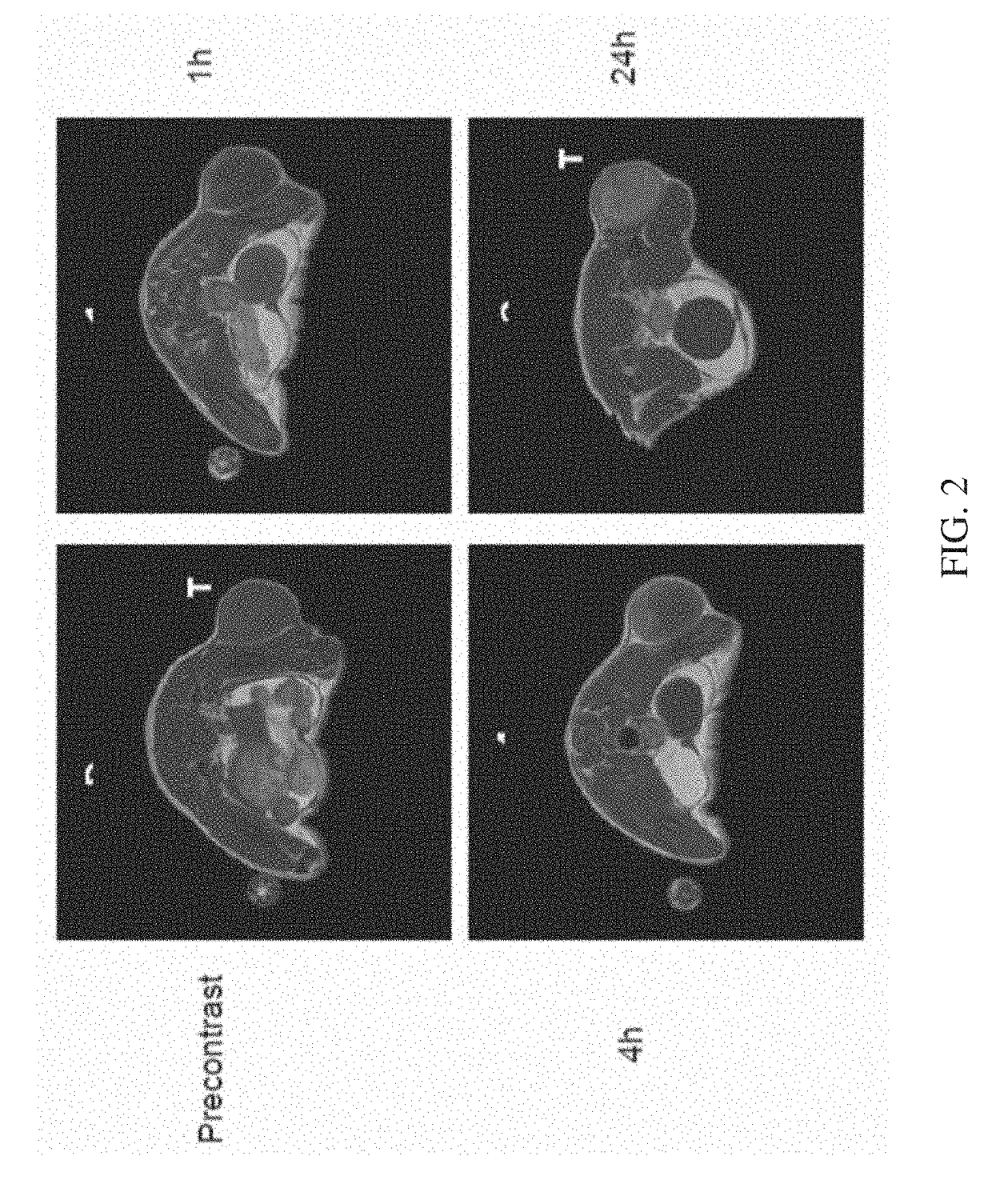
[0121]In this example, we demonstrate the successful in vivo MRI imaging of a tumor, using Gd-DO3A-404 as the MRI contrast agent. The data demonstrates that the backbone phospholipid and chelating agent are taken up and retained by solid tumors, demonstrating that such chelates incorporating various radioactive metals, as disclosed herein, would exhibit similar properties
[0122]For proof-of-concept in vivo imaging of tumor uptake of the Gd-DO3A-404 agent, nude athymic mouse with a flank A549 tumor (non small cell lung cancer) xenograft was scanned. The Gd-DO3A-404 agent (2.7 mg) was delivered via tail vein injection. Mice were anesthetized and scanning performed prior to contrast administration and at 1, 4, 24, 48, and 72 hours following contrast delivery. Imaging was performed on a 4.7T Varian preclinical MRI scanner with a volume quadrature coil. T1-weighted images were acquired at all imaging time points using a fast spin echo scan with the following pulse s...
example 3
ancer Imaging in Multiple Tumor Models
[0125]In this extension of Example 2, we demonstrate selective uptake and in vivo MRI imaging in two distinct flank tumor types, using Gd-DO3A-404 as the MRI contrast agent.
[0126]To test uptake and retention in rodent models of human cancer, flank xenografts were established in mice for two distinct tumor types, A549 (human non small cell lung cancer, NSCLC) and U87 (human glioma). N=3 for each model. For pre-contrast imaging, T1-W images of the tumor and abdomen (FIG. 4; 2 leftmost images) and T1 maps of the tumor were obtained.
[0127]At time zero (“contrast”), 2.5 mg of Gd-DO3A-404 (˜12 mmol / kg body mass) was delivered into the mice by intravenous injection. Animals were scanned pre-contrast and at various time points between one hour and seven days post-contrast (after one hour, 24 hours, 48 hours, three days, four days and seven days). T1 maps of the tumor were acquired for each time point, along with T1-weighted images of the tumor and the a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Time | aaaaa | aaaaa |
| Time | aaaaa | aaaaa |
| Time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com