Intracellular translation of circular RNA
a technology of rna and circular rna, which is applied in the direction of viruses/bacteriophages, biochemistry apparatus and processes, genetic material ingredients, etc., can solve the problems of inability to produce complete and active rna via in vitro transcription, cell death via apoptosis, and inability to overcome the difficulties of considering technical difficulties, so as to facilitate the formation of circular rna, the effect of increasing stability and resistan
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
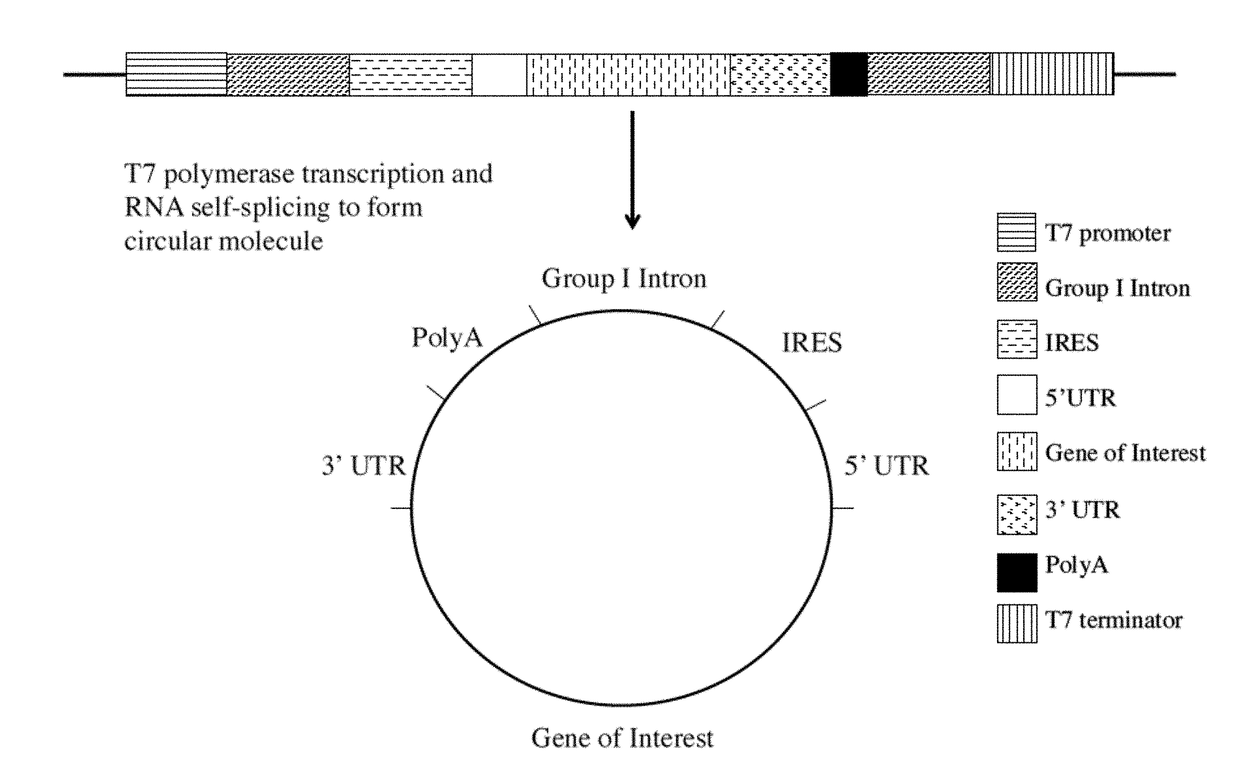
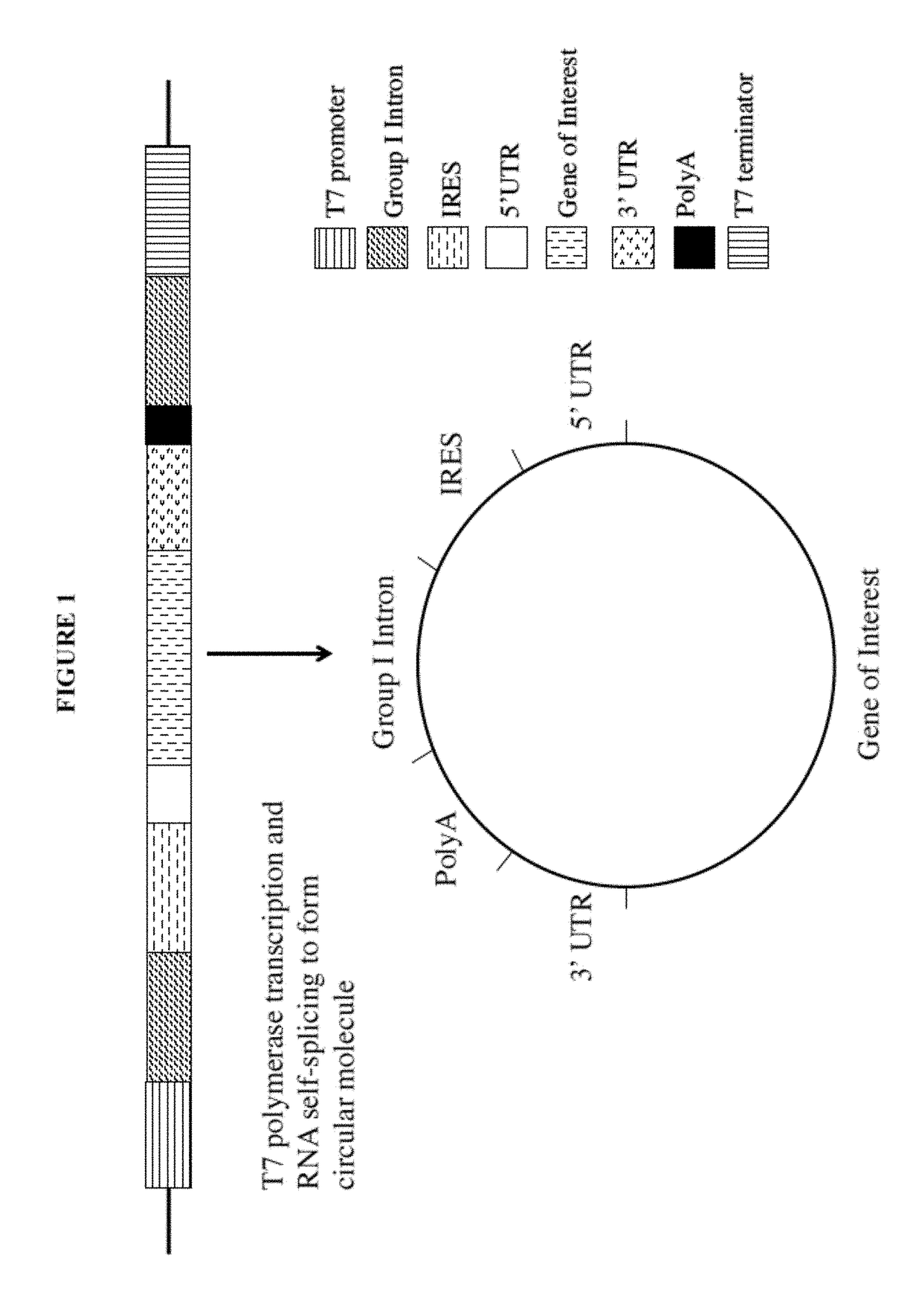
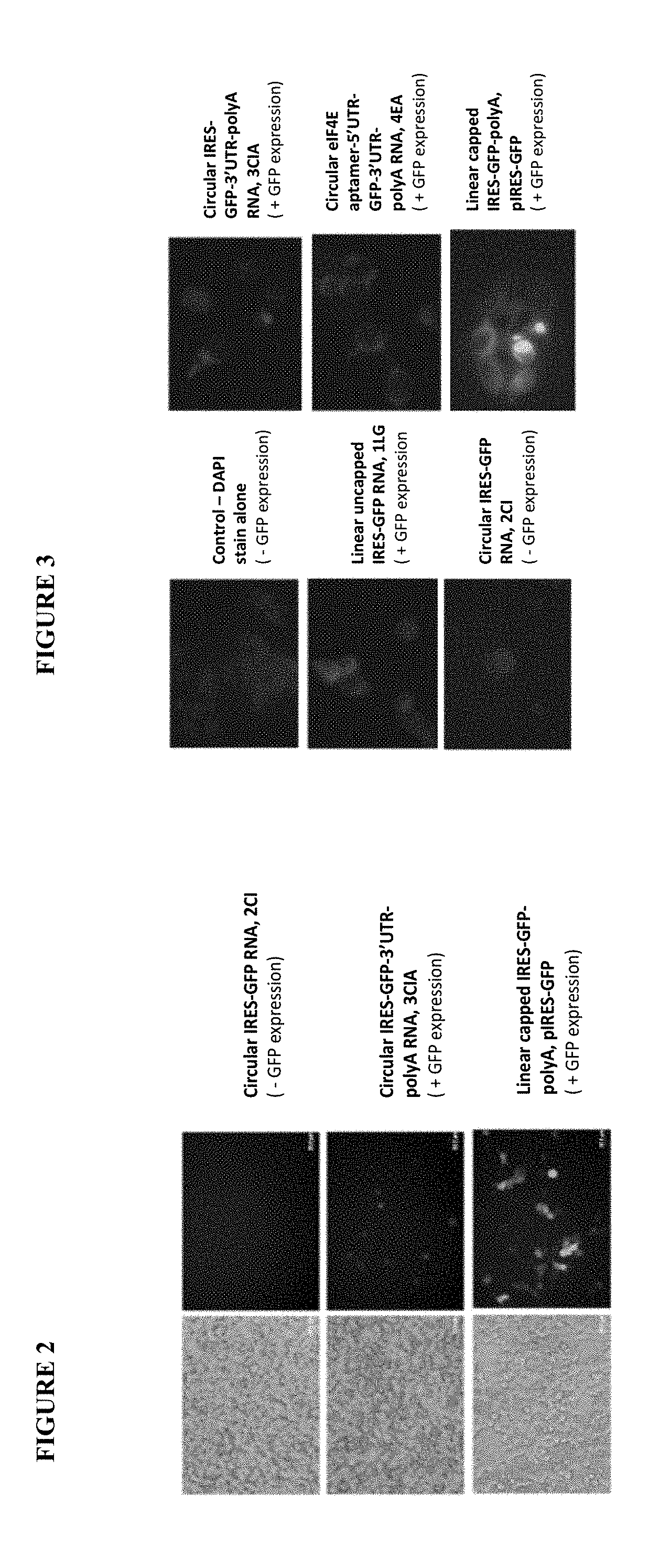
[0078]The current disclosure describes circular mRNA molecules that can successfully translate inside mammalian cells, as well as methods of making same, vectors for making same, and methods of using either the vector or the circular mRNA.
[0079]The circular mRNA features additional regions beyond the IRES and ORF in order to help recruit ribosomes to the circular mRNA. The circular mRNA has an IRES site, an ORF for protein of interest, a 3′UTR, and an optional polyA track. In some embodiments of the invention, there can be both an IRES and a 5′ UTR, depending upon how the IRES functions. Note that given the wide diversity of IRES sequences in nature, there will be a wide range of translational efficiencies when these IRES sequences are substituted in the proposed vector. In general, however, the invention will increase the efficiency of circular mRNA product regardless of the nature of the IRES in question because of the use of the polyA tail and 3′ UTR elements, both of which help ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com