Methods for growth and maturation of ovarian follicles
a technology of ovarian follicles and growth methods, which is applied in the direction of viruses/bacteriophages, drug compositions, and genetically modified cells, can solve the problems of difficult isolation and characterization of these cells from differentiating escs, and achieve the effect of promoting the growth and maturation of mammalian oocytes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Follicle-Like Structure Formation in Differentiating ESC Cultures
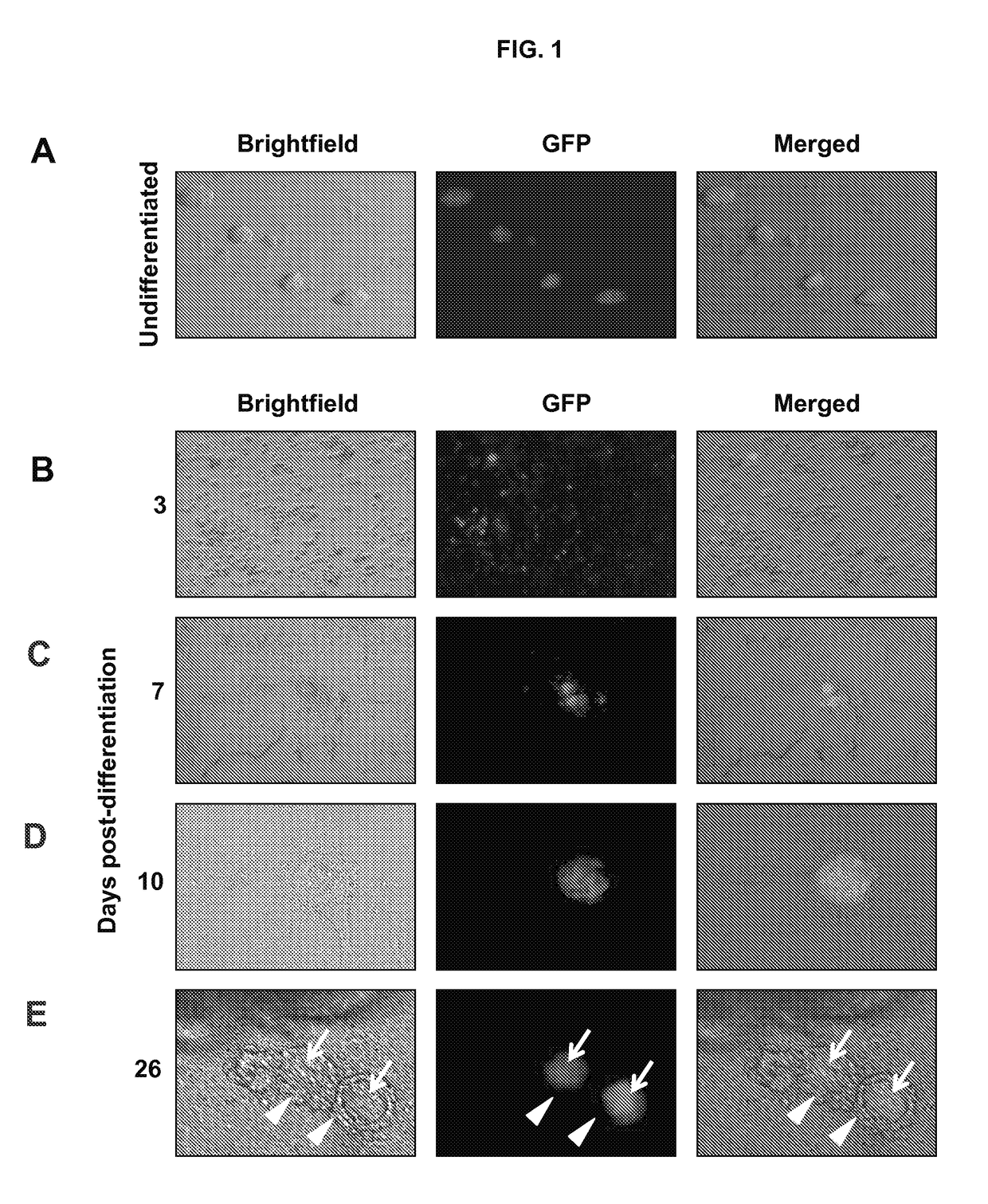
[0111]This example shows that differentiation of ESCs leads to the formation of follicle-like structures.
[0112]Materials and Methods
[0113]An undifferentiated mouse XX ESC line with germ cell lineage expression of green fluorescent protein (GFP) was generated from TgOG2 (ΔPE-Pou5f1-Gfp) transgenic female mice using standard methods, essentially as described in Hubner et al., Science, 300: 1251-56 (2003).
[0114]Undifferentiated TgOG2 ESCs were maintained on a mitotically-inactivated mouse embryonic fibroblast (MEF) feeder layer in DMEM supplemented with 15% heat-inactivated ESC-grade fetal bovine serum (FBS; Invitrogen, Carlsbad, Calif.), 1% sodium pyruvate, 1% non-essential amino acids, 1% penicillin-streptomycin (Invitrogen), 103 U / ml leukemia inhibitory factor (LIF; Millipore, Billerica, Mass.) and 0.1 mM β-mercaptoethanol (Sigma-Aldrich, St. Louis, Mo.). For differentiation, ESCs were separated from MEFs by differenti...
example 2
Granulosa Cell Specification and Isolation
[0119]This example shows a method for tracking and identifying granulosa cells that differentiate from ESCs.
[0120]Materials and Methods
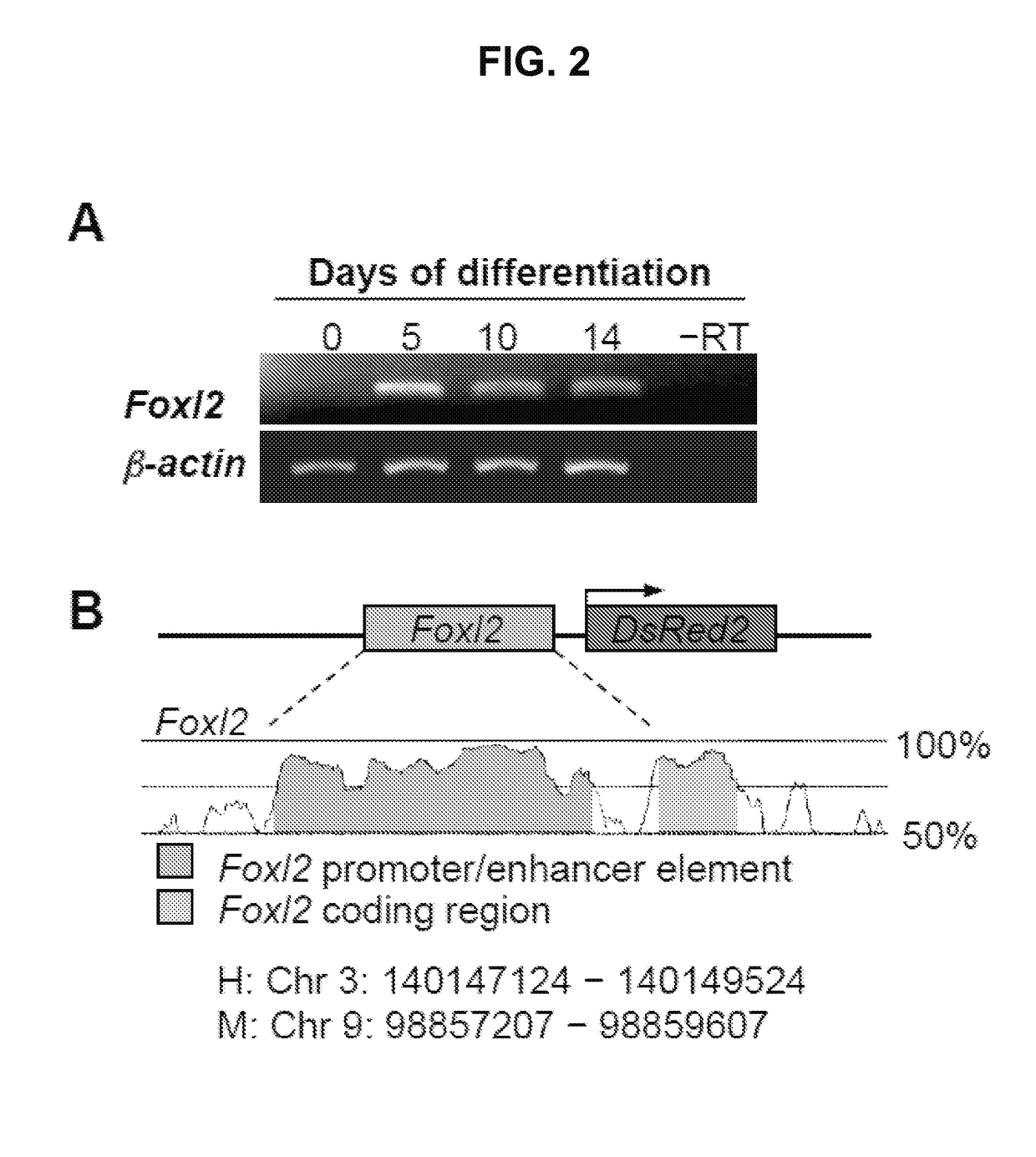
[0121]To identify and track ovarian somatic cells in differentiating ESC cultures, the expression of the early granulosa cell marker, Foxl2, in differentiating ESC cultures was mapped. The mapping revealed activation of the Foxl2 gene by day 5 (FIG. 2A). A 739 bp region of the Foxl2 gene promoter was identified using Genome Vista. The region was isolated from mouse genomic DNA and cloned into the pDsRed2-1 vector (Clontech, Mountain View, Calif.,) or the pLenti6 lentiviral construct containing the complete open reading frame of DsRed (Gateway Lentiviral System; Invitrogen), thus creating a DsRed expression vector under control of the Foxl2 gene promoter (FIG. 2B)
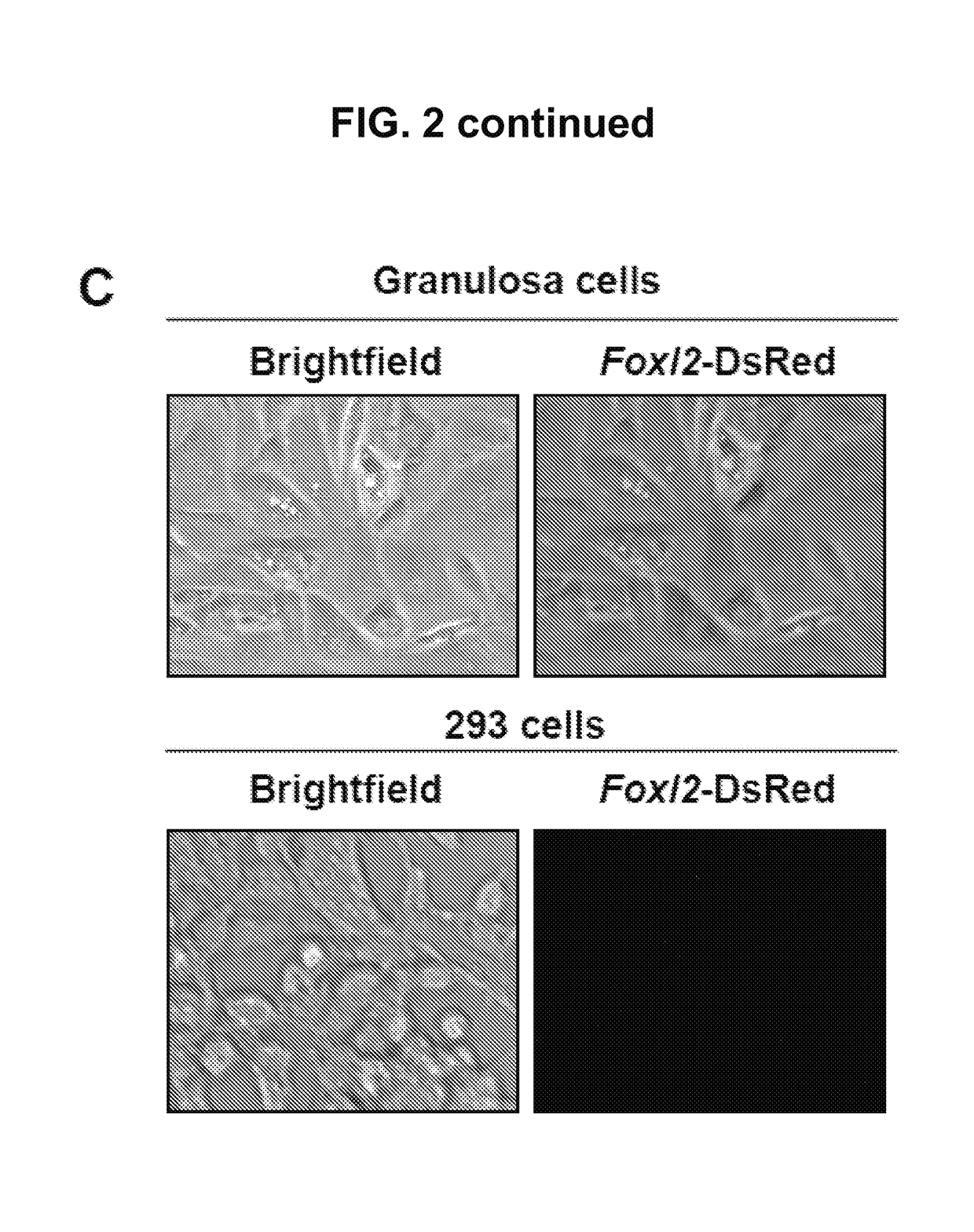
[0122]Promoter activity and specificity were verified using mouse granulosa cells as a positive control and 293 cells (Invitrogen) as a negative cont...
example 3
Intraovarian Transplantation of Granulosa Cells
[0131]This example shows granulosa cells derived from multi-potent cells migrate to immature oocytes and developing follicles in neo-natal ovaries.
[0132]Materials and Methods
[0133]Wild-type C57BL / 6 female mice (Charles River Laboratories, Wilmington, Mass., USA) were used in the following experiments.
[0134]Following differentiation of Foxl2-DsRed-expressing ESCs for 12 days, FACS was used to isolate DsRed-positive cells. For each experiment, 200-500 DsRed-positive cells were microinjected into a single neonatal (day 2-4 postpartum) wild-type mouse ovary using a Pneumatic PicoPump (World Precision Instruments, Sarasota, Fla.) (FIG. 6A-6B). Injected ovaries were then transplanted under kidney capsules of ovariectomized wild-type female mice at 6 weeks of age. At 8 days and 2 weeks post-transplantation, the grafted ovaries were removed and fixed in 4% paraformaldehyde (PFA) for analysis.
[0135]Fixed ovaries were embedded in paraffin, serial...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com