Therapeutic regimens for treatment of paroxysmal nocturnal hemoglobinuria
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
y Activity of Compound 1 in Combination with Eculizumab as Assessed Via a CAP-Mediated Hemolysis Assay with PNH Erythrocytes
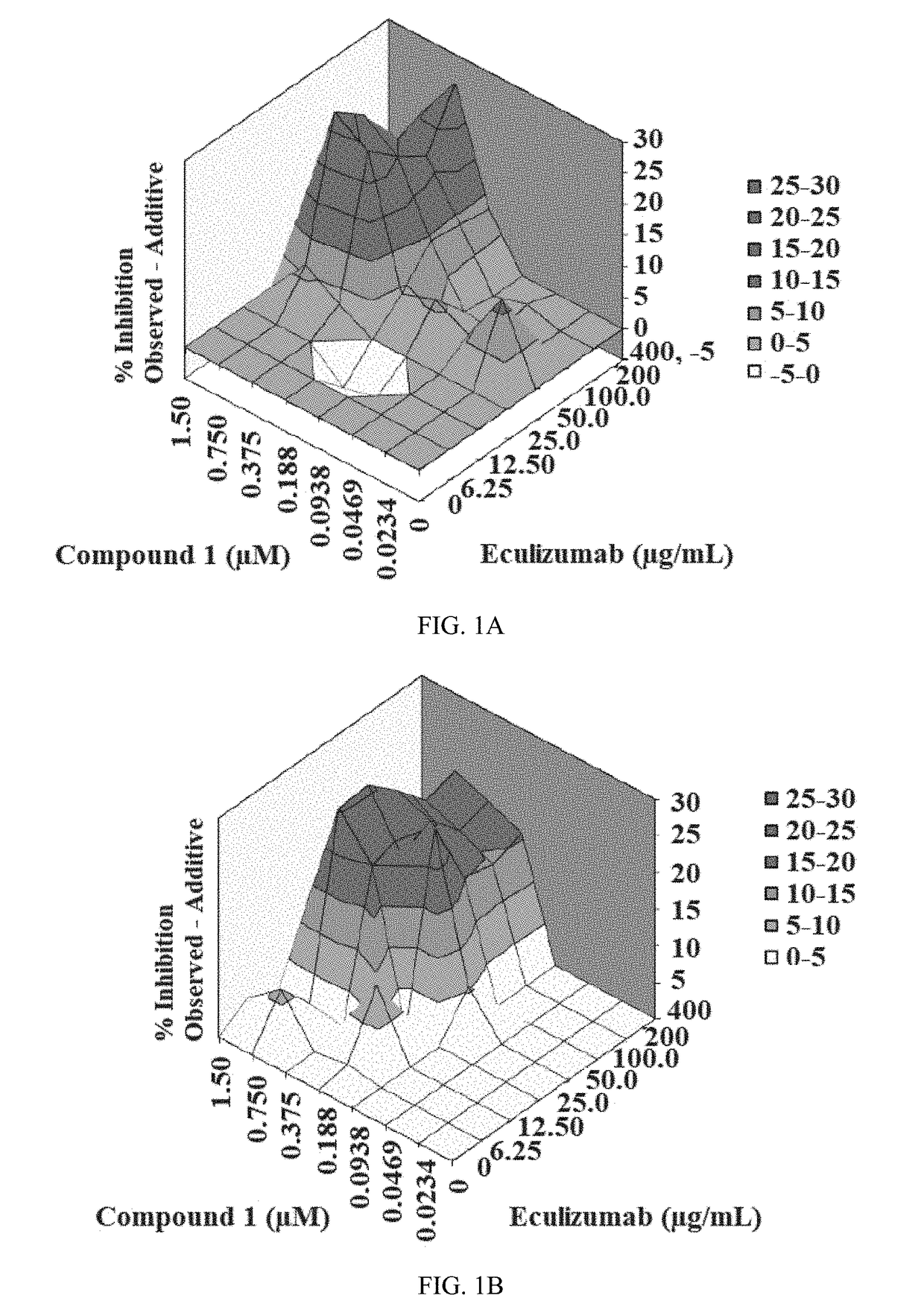
[0214]The inhibitory activity of Compound 1 and the humanized monoclonal antibody Eculizumab was assessed via a CAP-mediated hemolysis assay with PNH erythrocytes from subjects (described in Example 3). The CAP-mediated hemolysis assay with PNH erythrocytes was conducted two independent times and then analyzed by the method of Prichard and Shipman (described in Example 4). The inhibition of CAP activity for each experiment is shown in Table 2. The analytical results for each experiment, along with summary information, are shown in Table 3. The three-dimensional surface-graphs used in the analysis method of Prichard and Shipman are shown in FIGS. 1A-1B.
TABLE 2Inhibition of CAP activity of Compound 1 and EculizumabEculiz-Compound 1 (μM)umaba00.02340.04690.09380.1880.3750.751.5Inhibition (%), Experiment #140034373536567691972002531293155708898100122518234164879750...
example 2
y Activity of Compound 1 in Combination with Compstatin as Assessed Via a CAP-Mediated Hemolysis Assay with PNH Erythrocytes
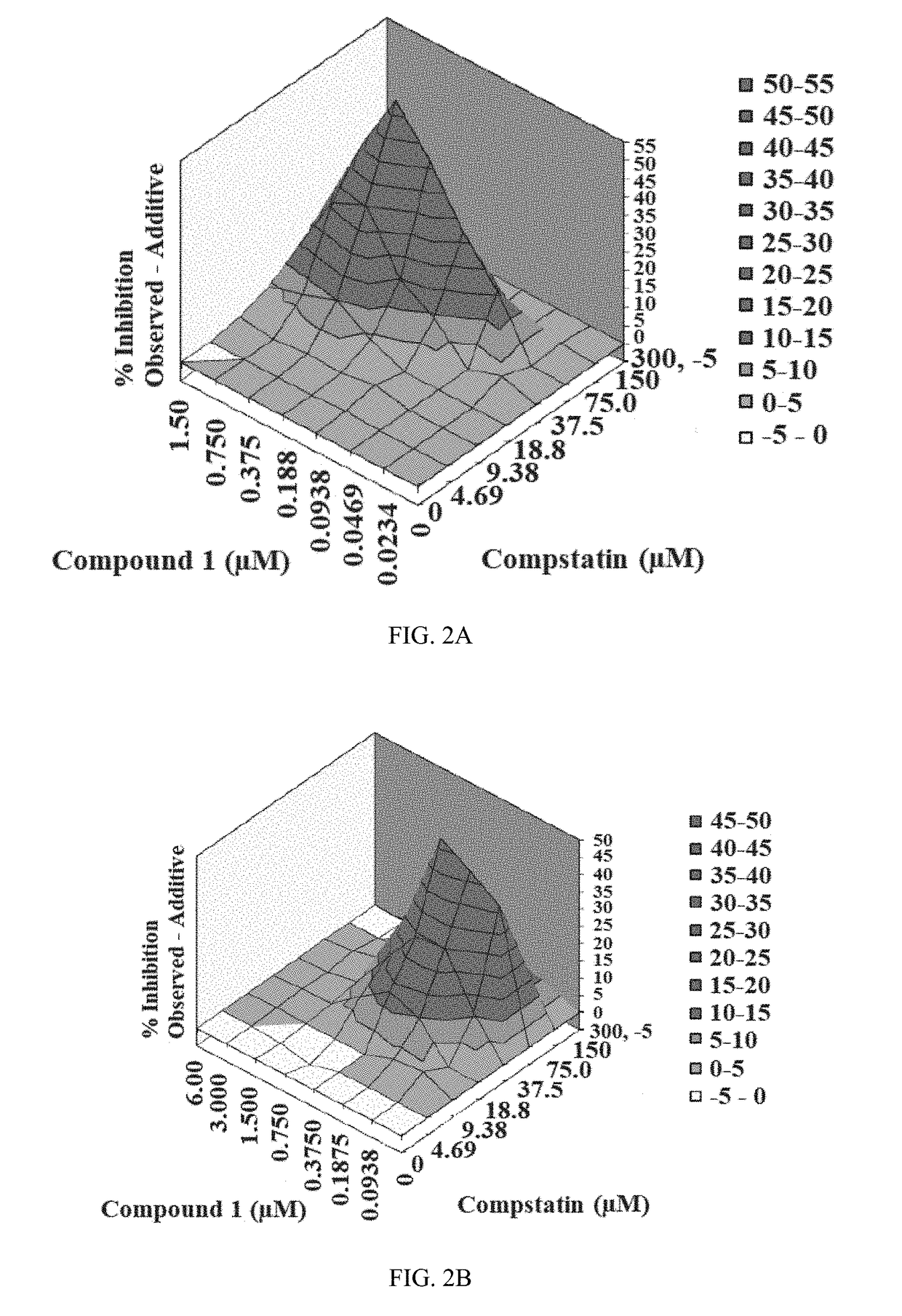
[0216]The inhibitory activity of Compound 1 and the complement C3 inhibitor Compstatin was assessed via a CAP-mediated hemolysis assay with PNH erythrocytes from subjects (described in Example 3). The CAP-mediated hemolysis assay with PNH erythrocytes was conducted two independent times and then analyzed by the method of Prichard and Shipman (described in Example 4) and by the method of Chou and Talalay (described in Example 5). The inhibition of CAP activity for each experiment is shown in Table 4. The analytical results for each experiment are shown in Table 5. The three-dimensional surface-graphs used in the analysis method of Prichard and Shipman are shown in FIGS. 2A-2B.
TABLE 4Inhibition of CAP activity of Compound 1 and Compstatin measured using the CAP-mediated hemolysis assay with PNH ErythrocytesComp-statin(μM)Compound 1 (μM)Inhibition (%), Experiment ...
example 3
ted Hemolysis Assay with Erythrocytes from PNH Subject A
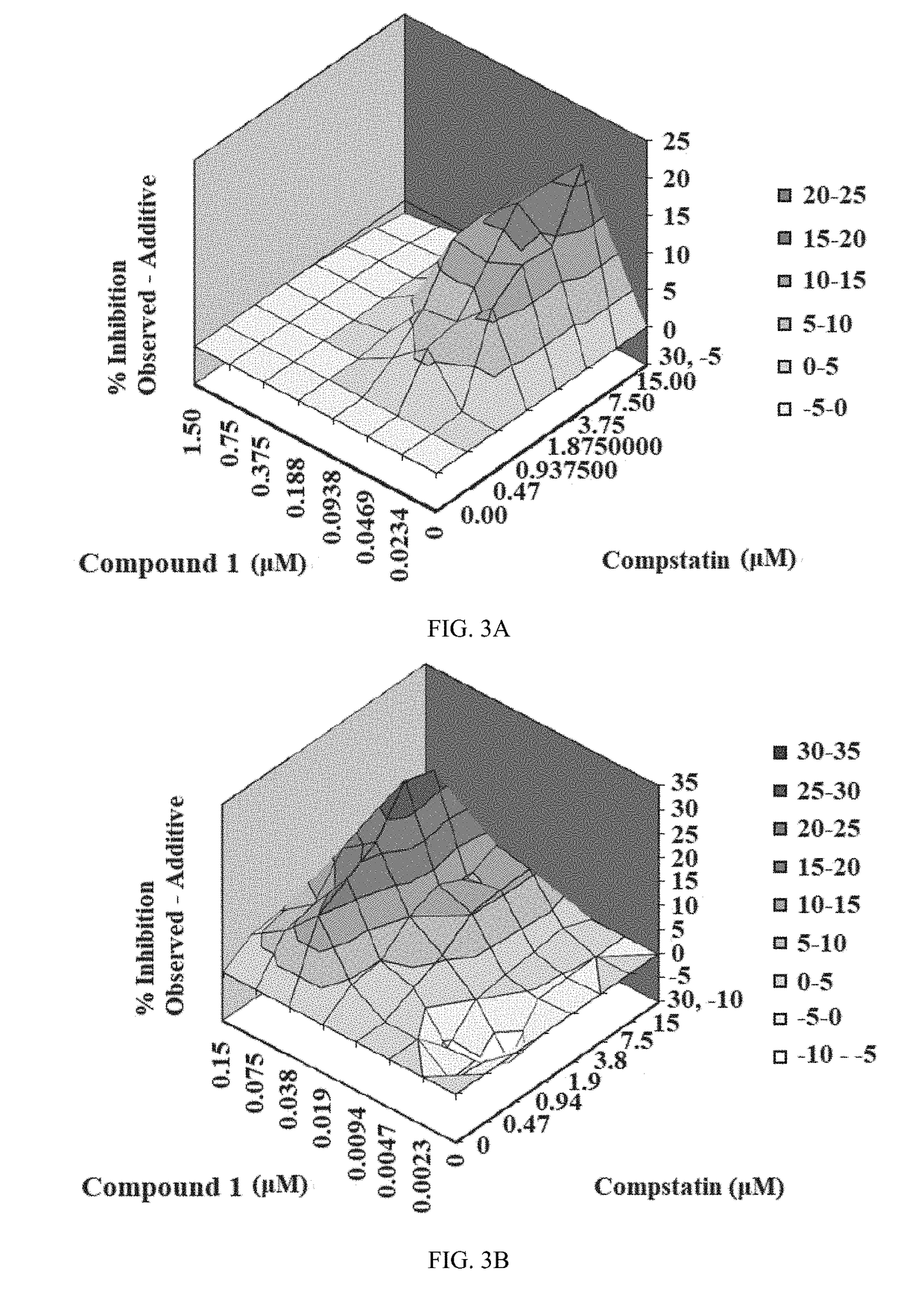
[0219]Combination studies of Compound 1 with Eculizumab (results are described in Example 1) and Compound 1 with Compstatin (results are described in Example 2) were performed using the CAP-mediated hemolysis assay with erythrocytes from PNH subject A and blood group ABO-compatible serum (NHS-AB, final assay concentration 20%). PNH Subject hematologic characteristics are shown in Table 6.
TABLE 6Characteristics of Subject AAge, Gender52, FBlood typeBErythrocyte clone size Type II / III (%)3.1 / 88Granulocyte clone size (%)99LDH (U / L)293Hemoglobin (g / dL)9.8Direct Coombs C3PosDirect Coombs IgGNegAbsolute reticulocyte count (K / cu mm)288.3Start of Eculizumab useStarted March 2011
[0220]Compound 1 and Compstatin were prepared as 15 mM stocks in DMSO. Eculizumab was obtained as a 10 mg / mL stock in buffered saline. Complement-preserved normal human serum (NETS) from an individual donor of ABO blood group type AB (NHS-AB) was purchase from B...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Time | aaaaa | aaaaa |
| Time | aaaaa | aaaaa |
| Density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com