Macro tissue explant, methods and uses therefor
a micro-tissue and explant technology, applied in the field of micro-tissue explant, methods, can solve the problems of difficult study of mammalian organs, inability to fully recapture the architecture and function of a tissue, and general incompatibility of cultures with high-throughput systems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
ultivation System for Intestinal Tissue
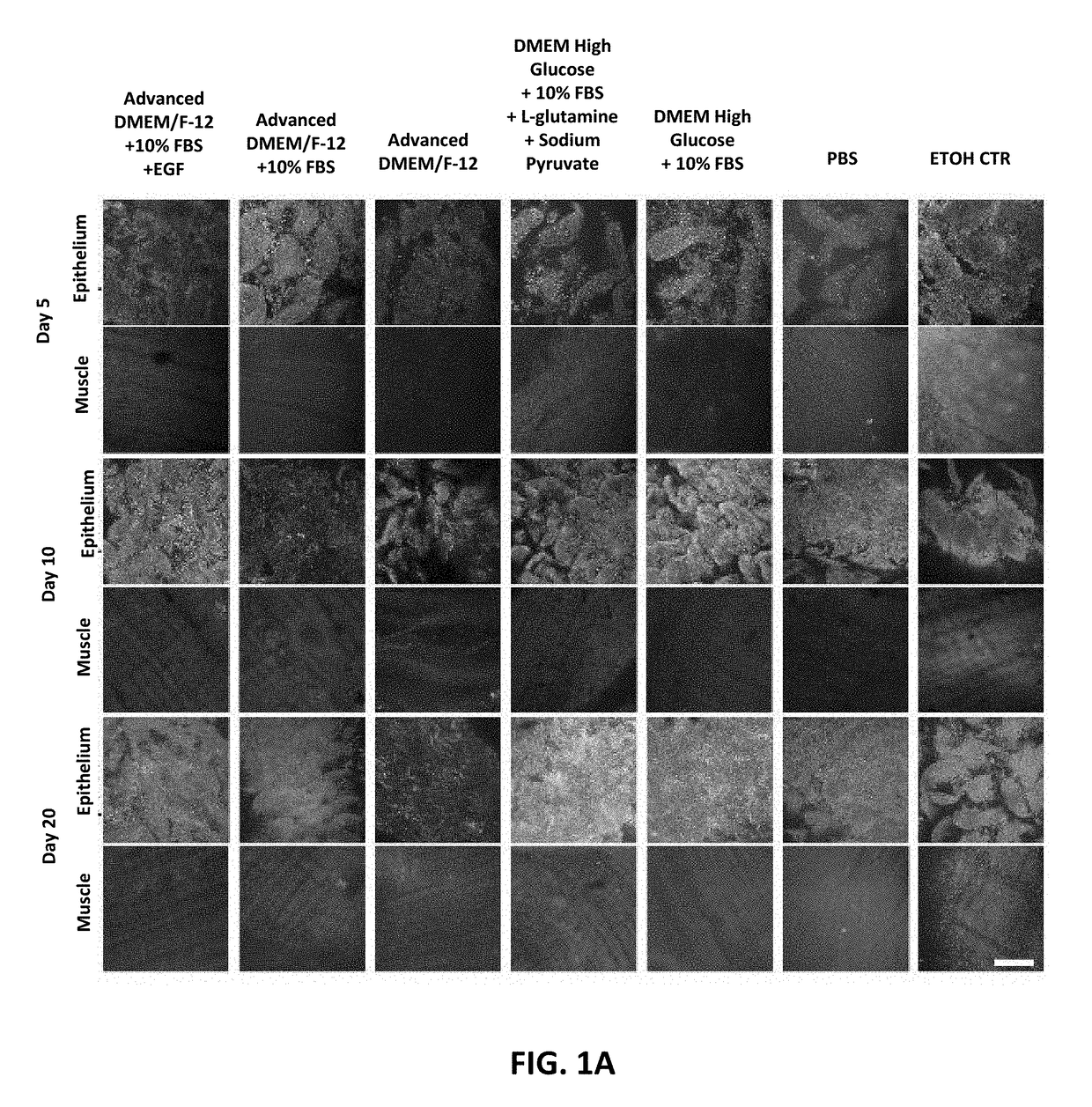
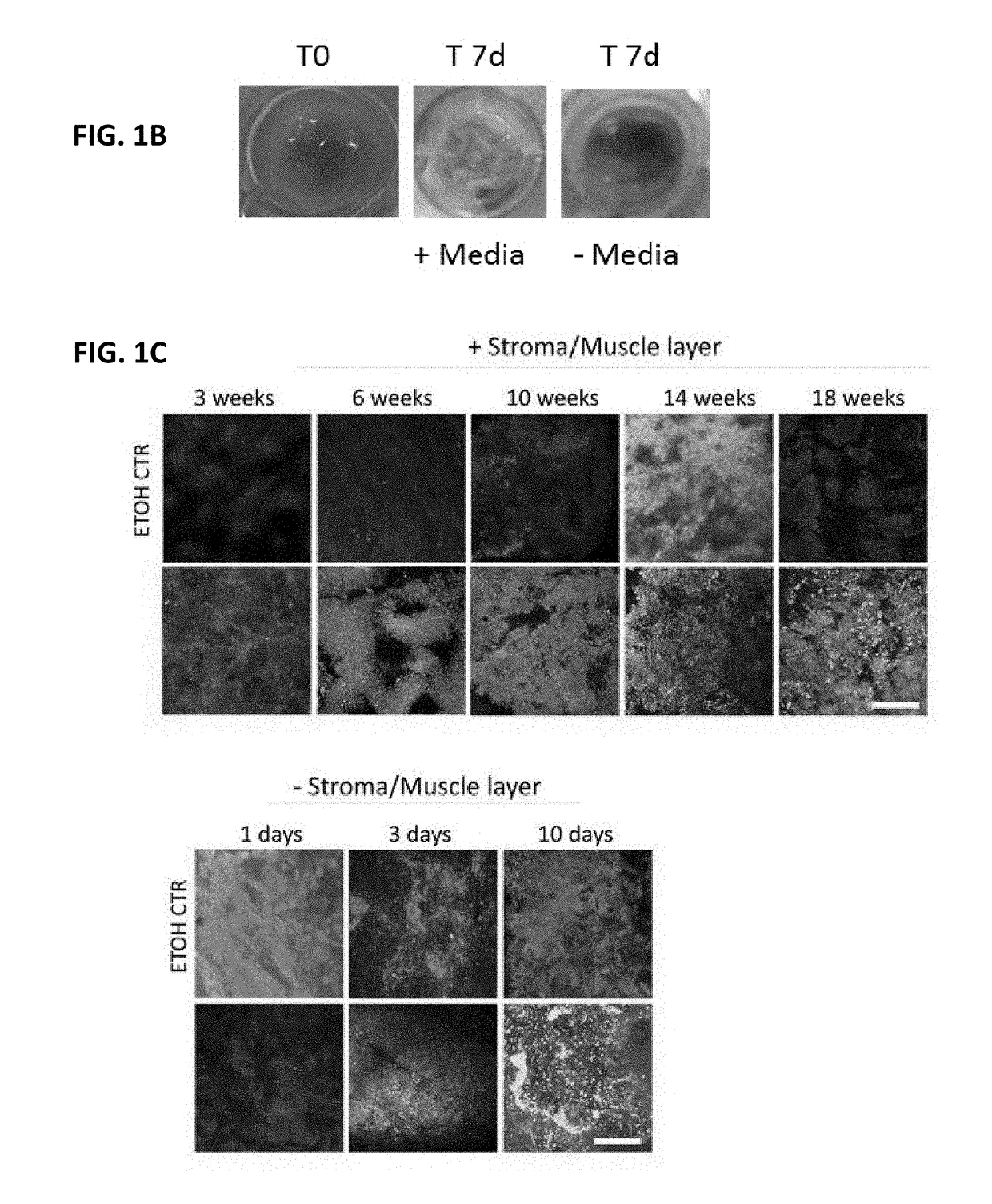
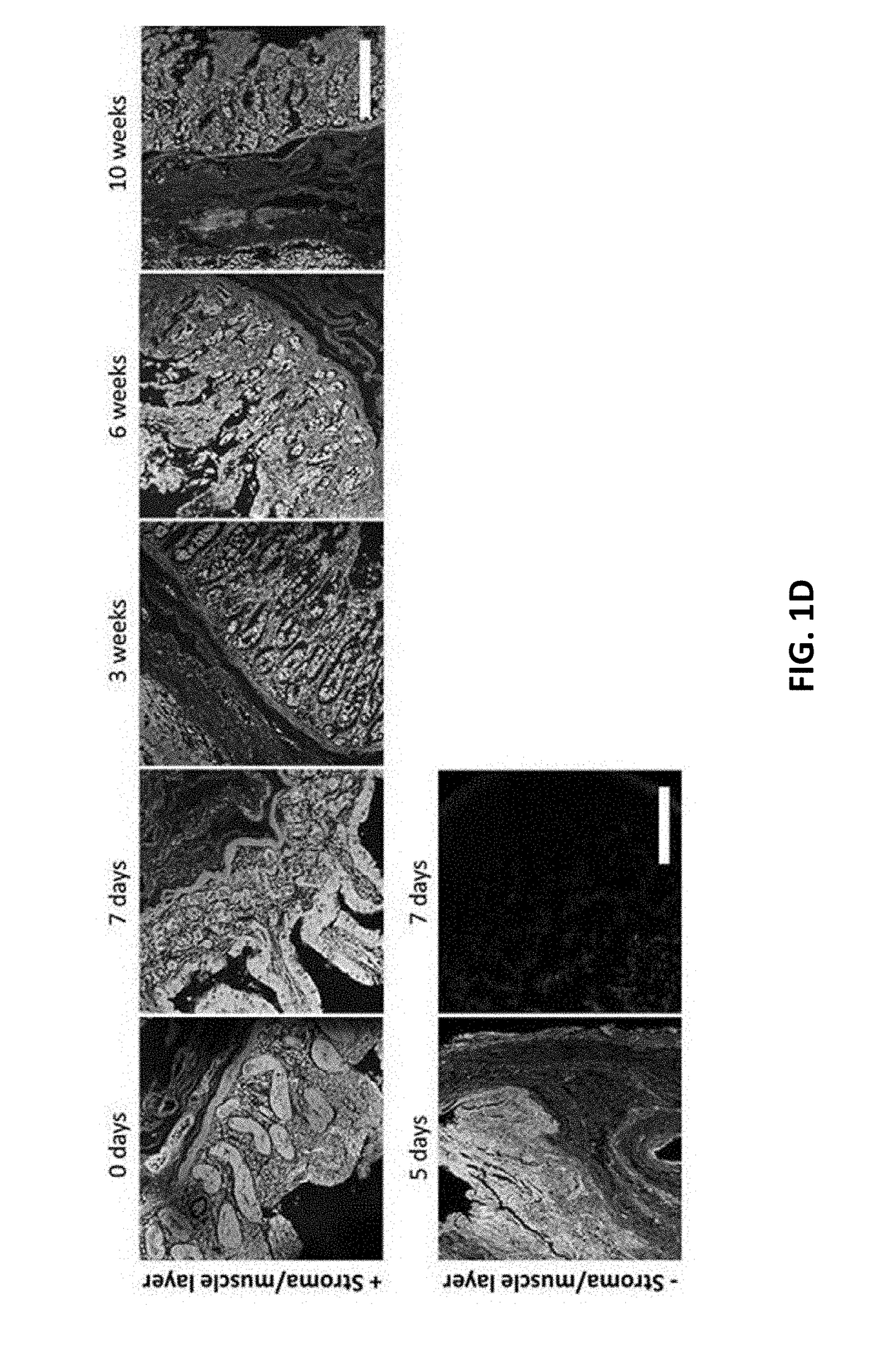
[0528]To determine whether intestinal tissue could be cultivated in a manner that maintained viability and in vivo architecture, small intestinal tissue was isolated from freshly procured intact gastrointestinal tract from pigs. The viability of intestinal tissue explants was found to be dependent on specific media compositions (FIG. 1A). No difference in viability was observed between tissue cultured in DMEM F12 alone and DMEM F12 with FBS or EGF added. In addition, FIG. 1B shows pictures of the luminal side of the small intestinal tissue explants with or without media cultivation after 7 days, indicating the explants survived best with media. The stroma was found to be essential in maintaining the cell survival of the intestinal epithelium in ex vivo cultivation (FIG. 1C). Confocal analysis of sectioned intestinal tissue explants that were stained with Dapi (blue, cell nucleus), Phalloidin (green, F-actin, Wheat Germ Agglutinin (Plasma membra...
example 2
l Tissue Explant Platform Development
[0537]Next, the use of the intestinal tissue explant from Example 1 in a high-throughput platform was investigated. An interface platform for intestinal tissue explant cultivation that enabled high-throughput intestinal drug perfusion measurements combined with long-term tissue cultivation capability was designed. Specifically, a broad range of different designs and materials for potential interfacing systems was systematically assessed. As shown in FIG. 2A, a system that enables low sample variability, tissue viability maintenance, rapid assembly and compatibility with robotic handling was developed. The design consisted of an upper device that compartmentalized the intestinal tissue in a 96 multi-well plate format. The tissue formed the bottom of the multi-well plate and was sealed off around each of the 96 wells by using an additional device underneath the tissue. The system was enclosed by a case that enabled adjustable pressure to maintain t...
example 3
l Tissue Explant System Intestinal Absorption Validation
[0546]The Food and Drug Administration (FDA), recommends using drugs approved for oral administration with human clinical pharmacokinetic data to validate the in vivo predictability of an in vitro intestinal perfusion system (Waiver of In Vivo Bioavailability and Bioequivalence Studies for Immediate-Release Sold Oral Dosage Forms Based on a Biopharmaceutics Classification System, U.S. Dep. Heal. Hum. Serv. Food drug Adm. Cent. Drug Eval. Res., 2000). Therefore, to confirm the use of the intestinal tissue explant as a system for predicting intestinal absorption, the perfusion of 60 model drugs was analyzed. Specifically, drugs from the 4 Biopharmaceutical Classification System (BCS) classes (16 BCs class I, 13 BCS class II, 15 BCS class III, and 12 BCS class IV), along with 4 dextran-based control substances, were used. The intestinal perfusion data obtained from the intestinal tissue explant system was compared to human intesti...
PUM
| Property | Measurement | Unit |
|---|---|---|
| time lapse analyses | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
| body mass | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com