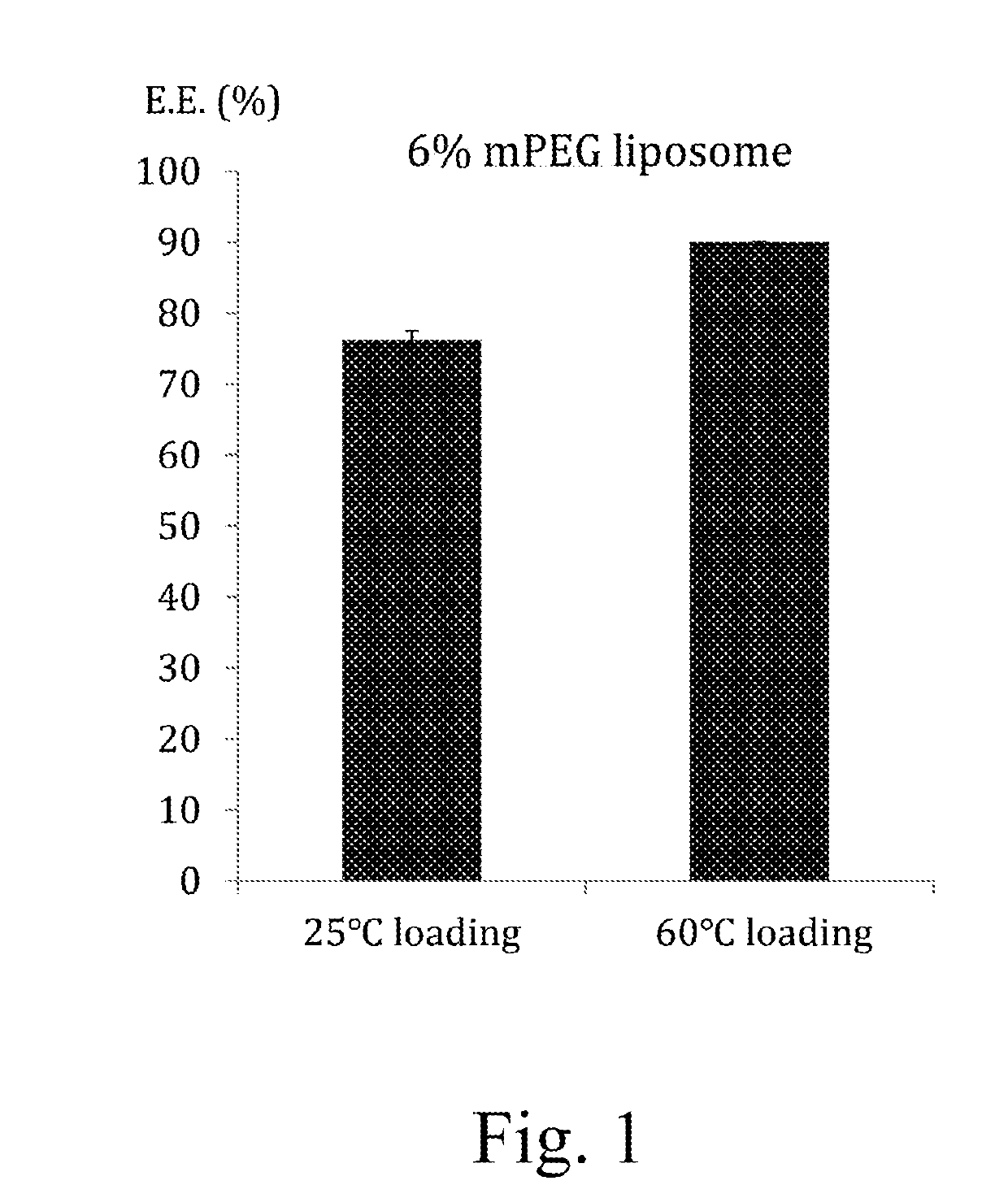
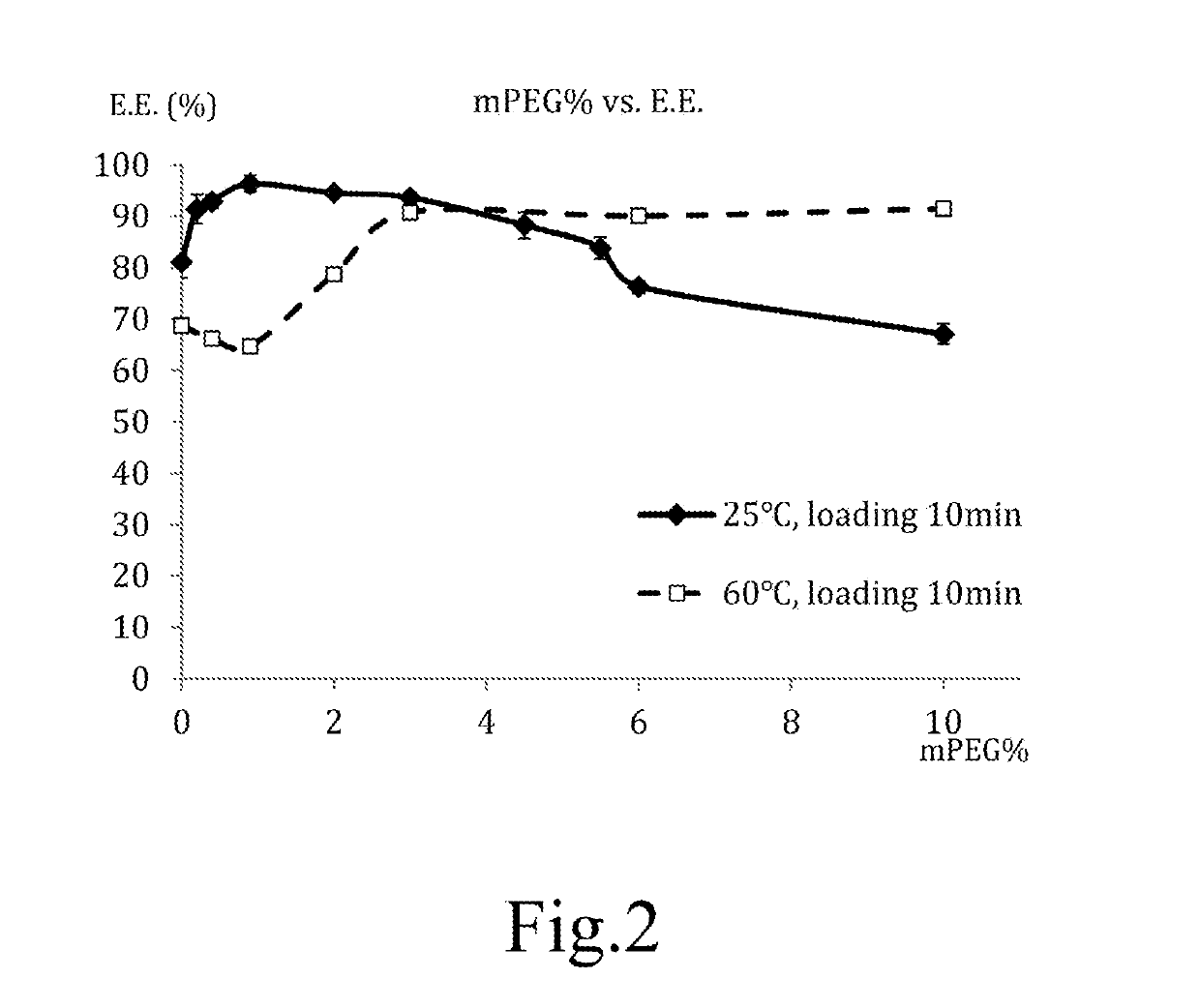
Liposomal composition containing mild acidic active agent
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Process for Preparing Lyophilized Cake
[0073]Cryoprotectant was dissolved in hot water (50˜60° C.) and then cooled to below 40° C. to form a cryoprotectant solution. Prostaglandin E1 was dissolved in tert-Butyl alcohol (TBA) being pre-warmed to liquefy the solvent at 40° C. and then transferred to and mixed with the cryoprotectant solution to form a prostaglandin solution. The prostaglandin solution was subjected to aseptic filtration and filled into 6R vial, followed by lyophilization to form a lyophilized cake containing PGE1 (hereby also denoted as alprostadil cake). The lyophilization was performed with a shelf lyophilizer under a program with the parameters as listed in the below Table I.
TABLE IThe lyophilization parameters for alprostadil cakeFreezePrimary dryingSecondary dryingEndtemperature (° C.)−40−40−30304duration (min)902403000720—pressure (mTorr)——100100100
example 2
Preparation of Alprostadil Cake with Various Components
[0074]To find a suitable formulation for forming the alprostadil cake according to the present disclosure, 52.6 μg PGE1 per vial was proposed for alprostadil cake, 125 mg cryoprotectant was used for our formulation.
[0075]As PGE1 was used as API in the formulation, a suitable solvent was needed to dissolve water-insoluble PGE1. PGE1 is soluble in tert-Butyl alcohol (TBA). TBA was selected to dissolve PGE1 to form the prostaglandin solution.
[0076]PGE1 undergo dehydration to form prostaglandin A1. In order to increase the shelf life of PGE1, lyophilization was used to reduce the moisture content to a very low level to minimize the degradation. Screening of the candidate formulations were based on results of 40° C. accelerated stability test.
[0077]As the content of cryoprotectant and PGE1 per vial was fixed as above, the other lyophilization parameters, such as water content, TBA addition or others were further modulated as describe...
example 2a
Effect of Water Content in the Prostaglandin Solution
[0078]First, upon the fixed percentage of TBA, the effect of percentage of water before lyophilization was investigated by using the formulations as listed in Table III to form the alprostadil cakes.
TABLE IIIFormulations of the prostaglandin solutions with various water contentComposition per vial: mg (% (w:w))FormulationlactoseCodePGE1*TBAmonohydratewaterPc0290.052613.8 (1.02)125 (9.26) 1211.1 (89.71)(0.0039)Pc0300.052610.8 (1.02)125 (11.90) 914.2 (87.07)(0.0050)Pc0310.0526 9.2 (1.02)125 (13.89) 765.7 (85.08)(0.0058)Pc0320.0526 7.7 (1.02)125 (16.67) 617.3 (82.30)(0.0070)*52.6 μg instead of 50 μg of PGE1 was used hereby to adjust the final concentration of PGE1 after reconstitution by 5 mL liposome solution to be 10 μg / mL
[0079]As shown in Table IV, all formulations met the criteria of 90% PGE1 remaining after 6 weeks at 40° C., the remaining content decreased as percentage of water in the prostaglandin solution decreased. It demon...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com