Pharmaceutical composition for treatment and prevention of chronic disease
a technology of pharmaceutical compositions and compositions, applied in the field of pharmaceutical compositions, to achieve the effect of low efficacy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
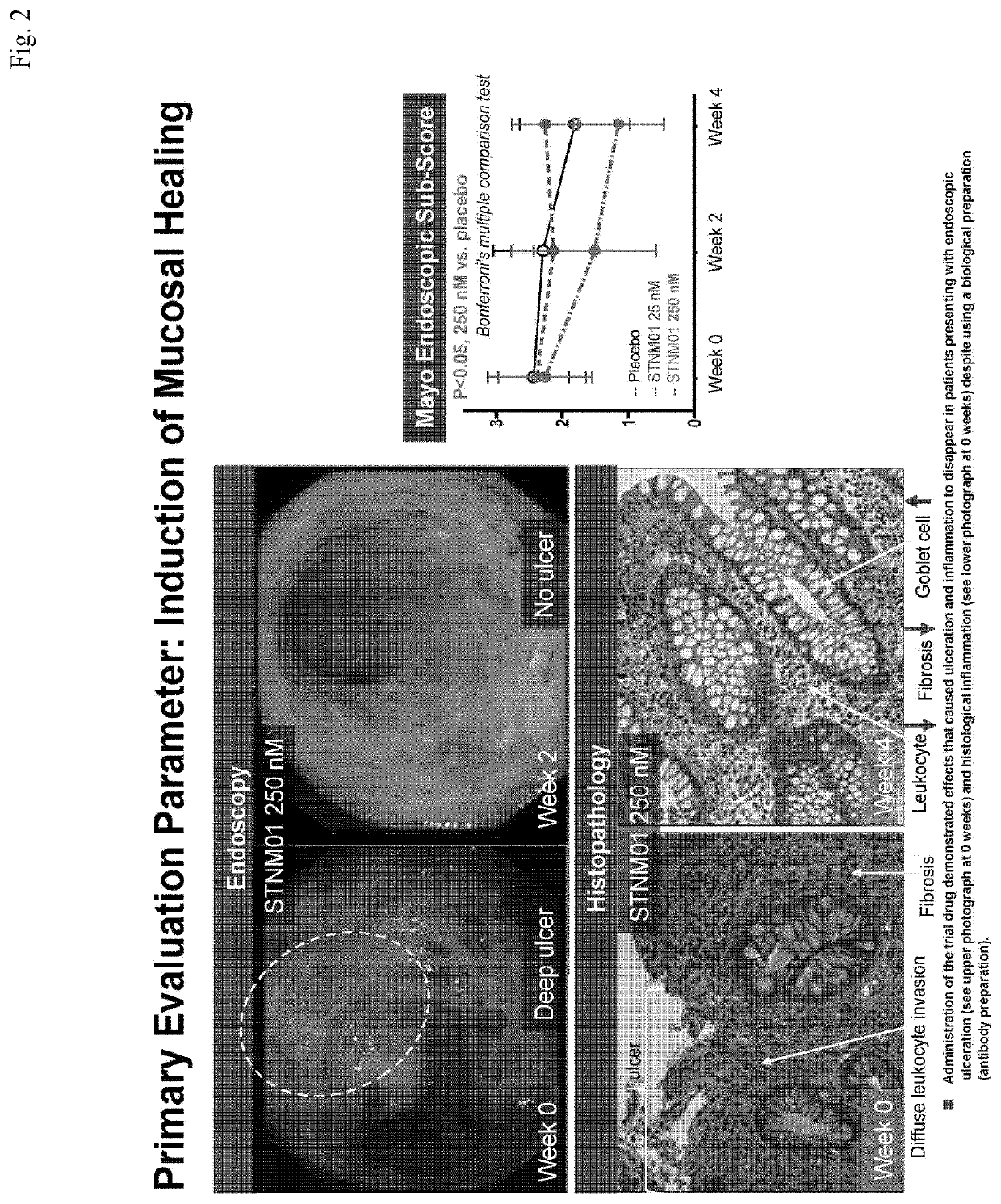
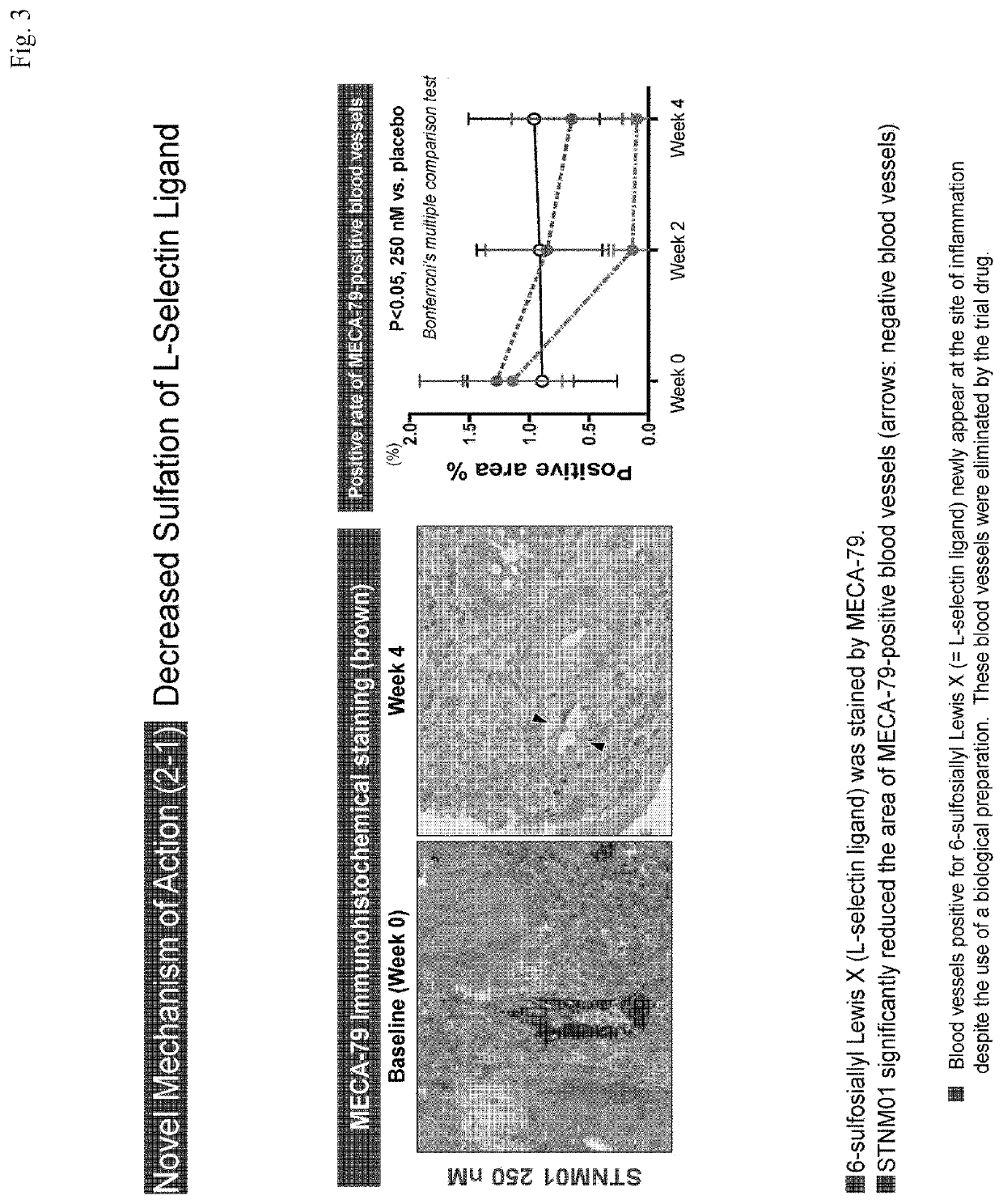
[0086]The following provides an explanation of a clinical trial of the pharmaceutical composition of the present invention based on Sections 8 and 9 of the phase IIa clinical trial protocol of STNM01. Furthermore, in the following explanation, although terms such as “will be” representing the future tense are used in the description of the trial protocol, the clinical trial was actually completed by the filing date of the present application.
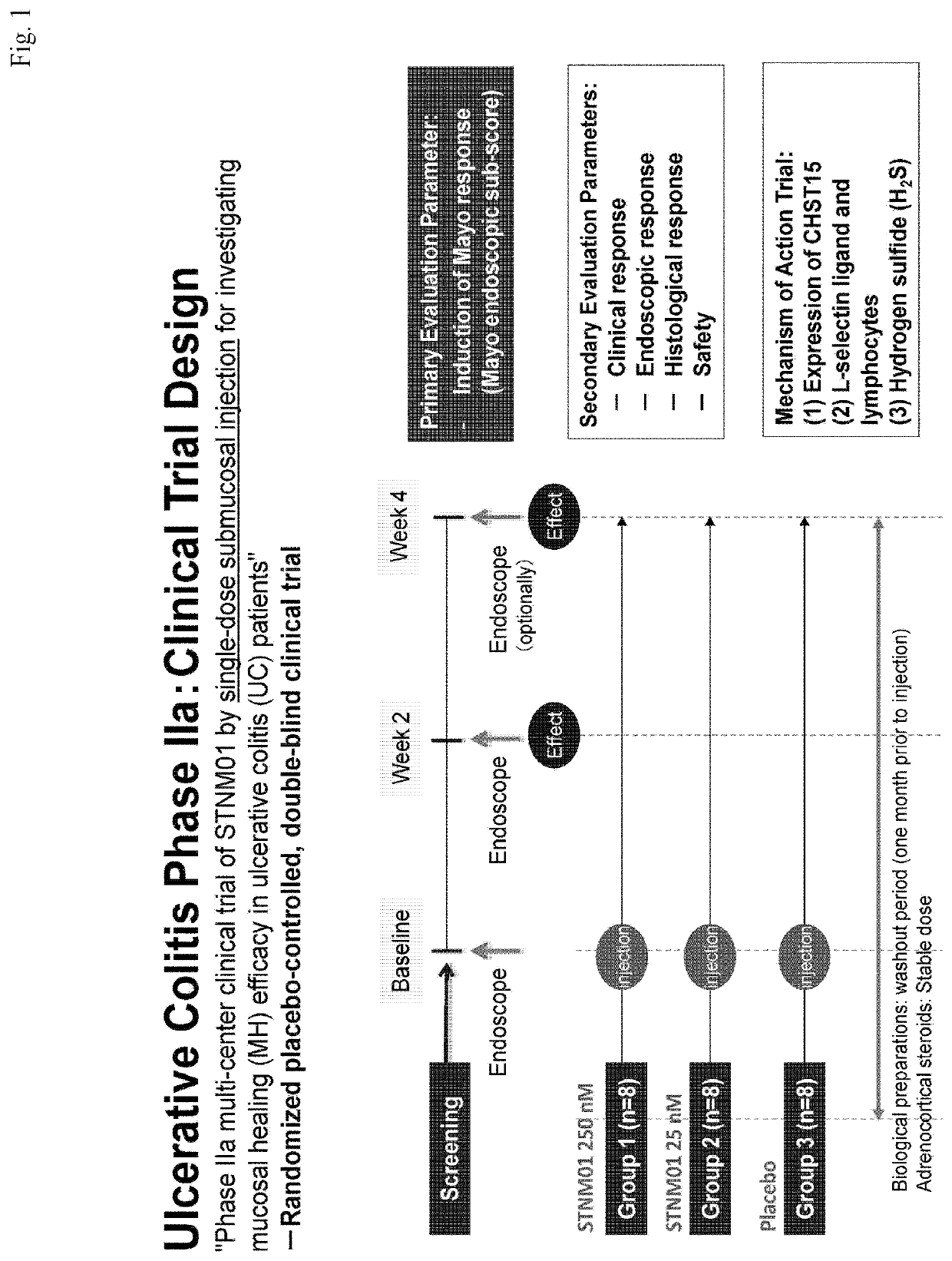
[0087]Clinical Trial Protocol Section 8—Trial Design and Scheduled Sample Size
[0088]8.1 Trial Design
[0089]This clinical trial is a randomized double-blind, placebo-controlled parallel-group trial of STNM01 by single-dose submucosal injection.
[0090]Freeze-dried STNM01 will be diluted using physiological saline. The trial drug will be administered submucosally into the rectosigmoid colon using an endoscope. The patients will be randomized so as to receive STNM01 (a concentrations of 25 nM and 250 nM) or a placebo. The subjects will receive a singl...
example 2
[0197]The present example provides an explanation of the results of a repeated-dose investigator initiated trial (IIT) in which biological preparation (Infliximab and / or Adalimunab)-resistant ulcerative colitis (UC) patients were administered the pharmaceutical composition of the present invention (STNM01).
[0198]FIG. 7 is a schematic diagram of the trial design of the investigator initiated trial of the present example. A total of five subjects observed to be resistant to a biological preparation (Infliximab and / or Adalimunab) in screening were administered 250 nM STNM01 by submucosal injection using an endoscope for a total of three times consisting of baseline (week 0) and in week 2 and week 4. In the trial of Example 1, subjects administered a biological preparation were administered STNM01 following a washout period during which time the biological preparation was not administered for one month. In the trial of Example 2, however, STNM01 was administered while continuing adminis...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Volume | aaaaa | aaaaa |
| Volume | aaaaa | aaaaa |
| Molar density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com