Binding molecule specific for lrig-1 protein and use thereof
a technology of lrig-1 protein and binding molecule, which is applied in the field of binding molecule specific for lrig1 protein, can solve the problems of no genes and proteins that can target only the regulatory t cells alone, and achieve the effects of regulating immunological tolerance, and reducing the number of treg cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
preparation example 1
[Preparation Example 1] T Cell Subset Cell Culture
[0154]In order to identify whether the Lrig-1 protein is expressed only in regulatory T cells (Treg), the subsets of T cells, Th0, Th1, Th2, Th17, and iTreg, were prepared. The iTreg refers to cells whose differentiation has been artificially induced in a medium containing the following composition, unlike nTreg which has been naturally isolated.
[0155]The subsets of the T cells were induced to differentiate into respective cells by first isolating naive T cells obtained from the spleen of mice, causing RPMI1640 (Invitrogen Gibco, Grand Island, N.Y.) nutrient medium that contains 10% fetal bovine serum (FBS; HyClone, Logan, Utah) to further contain the respective ingredients of Table 1 below, and performing 72-hour incubation in an incubator at 37° C., 5% CO2.
TABLE 1Differentiated cellCompositionTh0anti-CD3, anti-CD28Th1IL-12, anti-IL-4 antibodyTh2IL-4, anti-IFNββTh17IL-6, TGFββ, anti-IFNββ, anti-IL-4iTregIL-2, TGFββ
example 1
[Example 1] Structural Analysis of Lrig-1
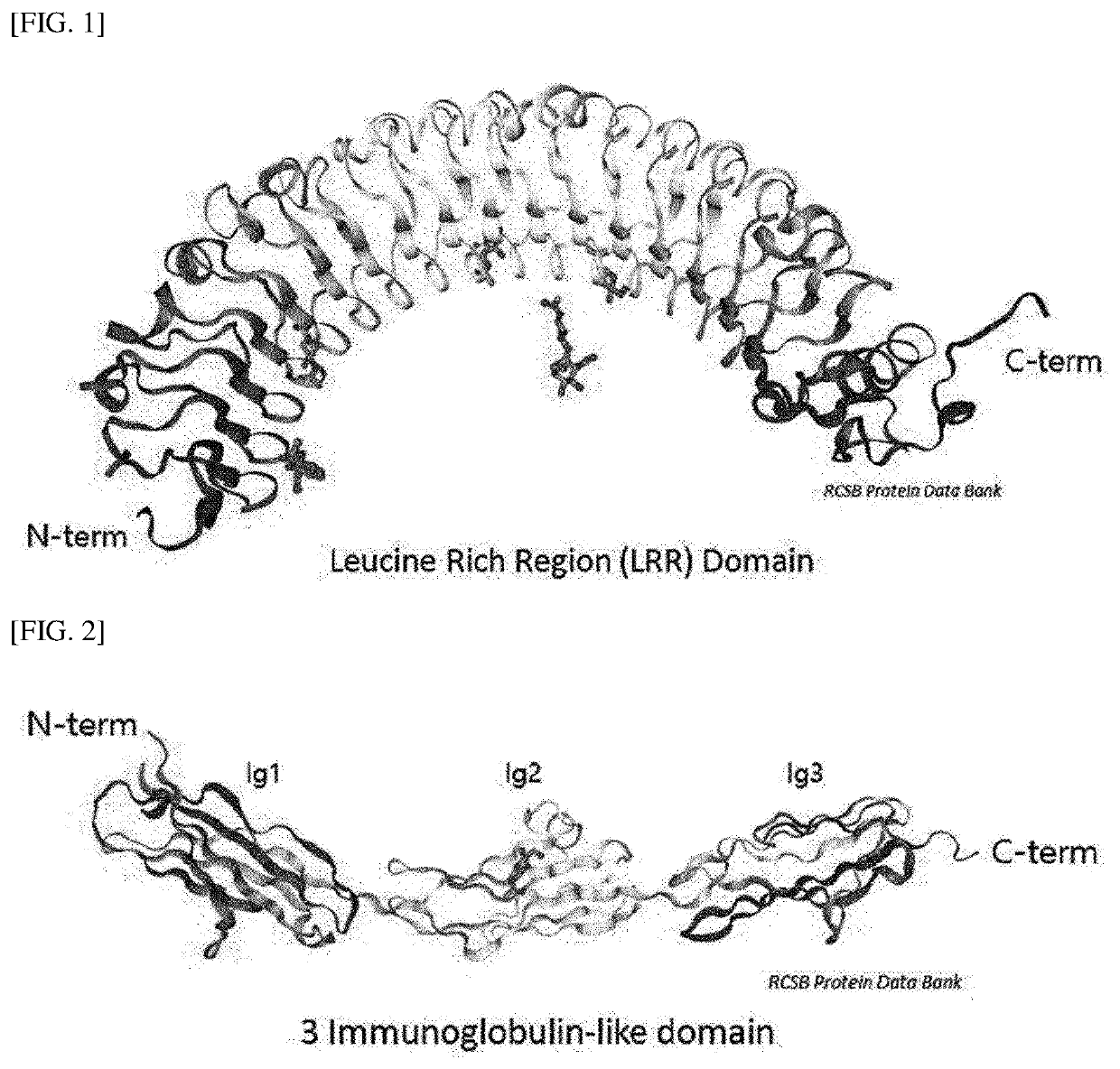
[0156]A three-dimensional steric structure of the extracellular domain of the Lrig-1 protein was predicted to produce antibodies specific for the Lrig-1 protein, a surface protein of regulatory T cells.
[0157]First, in order to predict base sequences of epitopes (epitopes), tools of Uniprot (http: / / www.uniprot.org) and RCSB Protein Data Bank (http: / / www.rcsb.org / pdb) were used to predict a three-dimensional steric structure of the extracellular domain (ECD) of the Lrig-1 protein so that the structure of ECD is identified. Then, the results are illustrated in FIGS. 1 and 2.
[0158]As illustrated in FIG. 1, a total of 15 leucine-rich regions of LRR1 to LRR15 existed in the Lrig-LRR domain (amino acid sequence at positions 41 to 494) in the extracellular domain of the Lrig-1 protein. Each of the LRR domains is composed of 23 to 27 amino acids, with 3 to 5 leucine being present.
[0159]In addition, as illustrated in FIG. 2, three immunoglobulin-like d...
example 2
[Example 2] Prediction of Lrig-1 Epitope Amino Acid Sequence
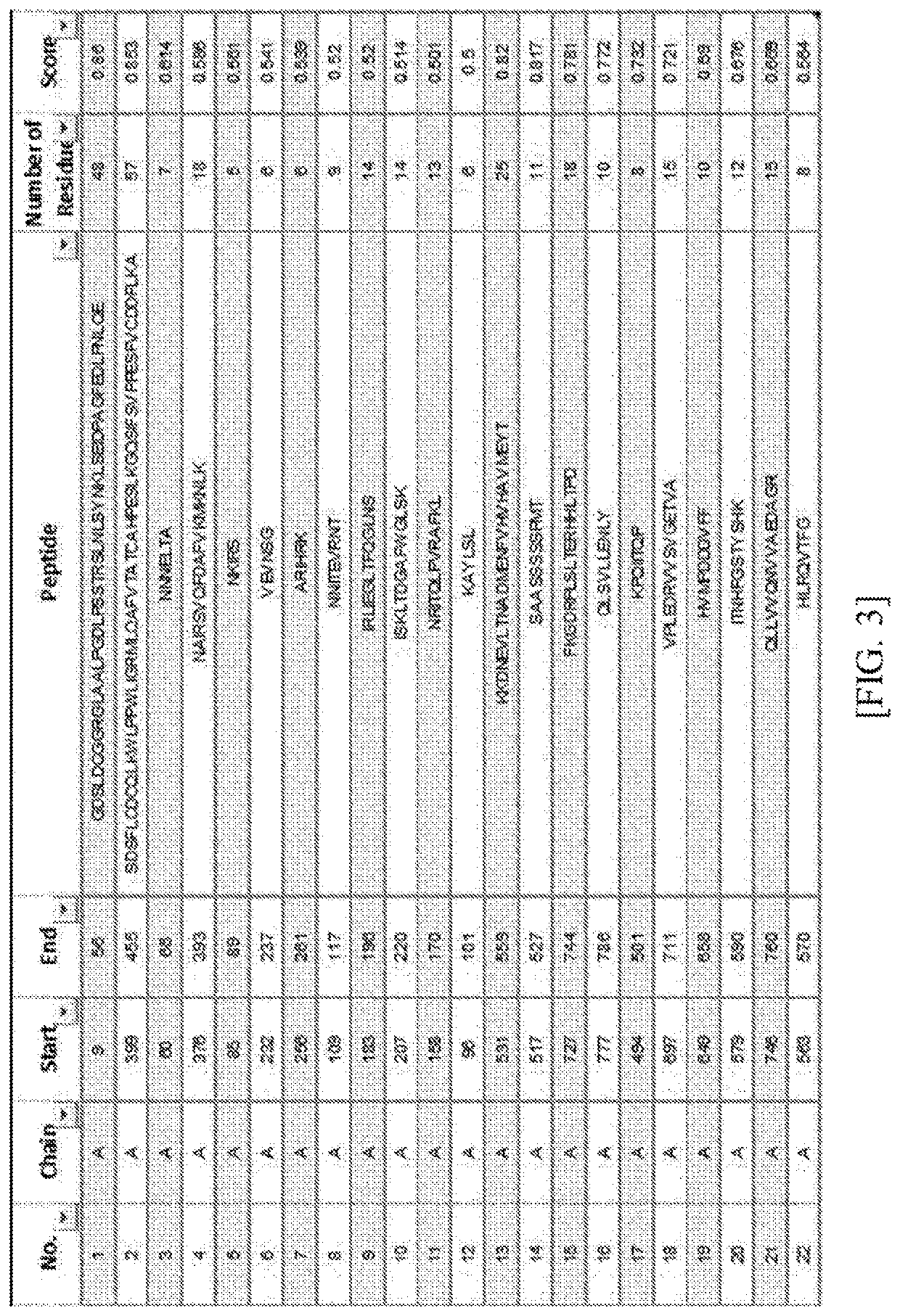
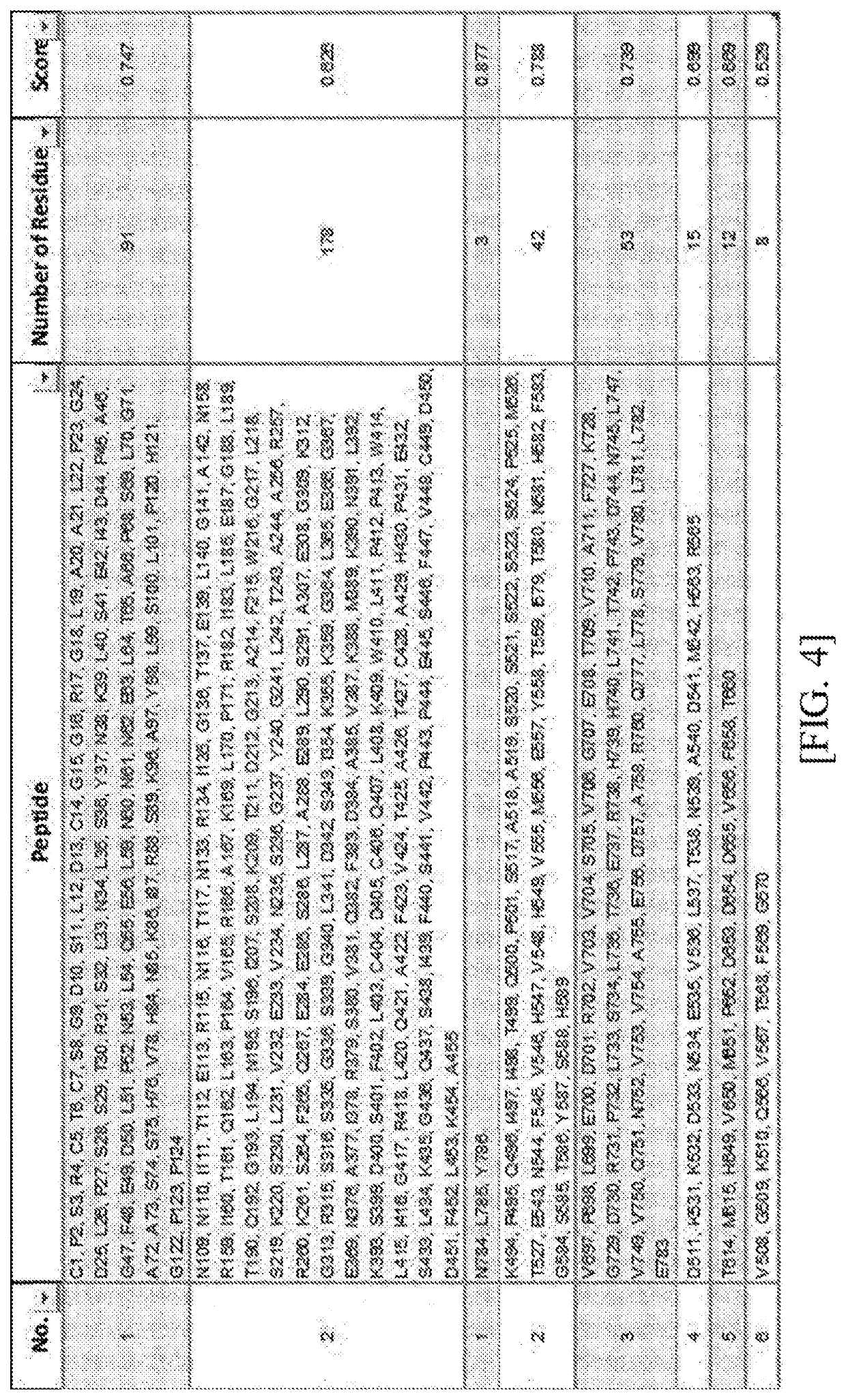
[0160]Prediction of the above base sequence was performed using Ellipro server (http: / / tools.iedb.org / ellipro / ) which is an epitope prediction software based on a structure of the Lrig-1 protein. The Ellipro search engine was used because it corresponds to a search engine known to be the most reliable among the existing algorithms for predicting an epitope.
[0161]The extracellular domain analyzed in Example 1 was entered into the epitope prediction software, and then predicted contiguous or discontiguous amino acid sequences of the predicted epitopes are illustrated in FIGS. 3 and 4.
[0162]As illustrated in FIGS. 3 and 4, a total of 22 contiguous epitope amino acid sequences were predicted, and a total of 8 discontiguous epitope amino acid sequences were predicted.
[Production Examples 1 to 4] Production of Monoclonal Antibodies Specific to Lrig-1 Protein
[0163]Antibodies specific for the Lrig-1 protein according to the present...
PUM
| Property | Measurement | Unit |
|---|---|---|
| structure | aaaaa | aaaaa |
| affinity | aaaaa | aaaaa |
| binding affinity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com