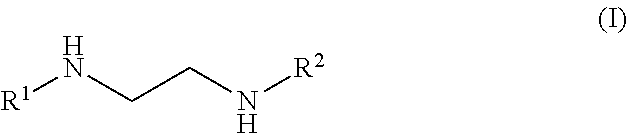
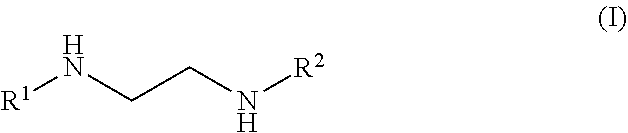
Plating bath composition for electroless plating of gold and a method for depositing a gold layer
a plating bath and electroless technology, applied in the direction of metallic material coating process, liquid/solution decomposition chemical coating, coating, etc., can solve the problems of insufficient stability of gold plating bath, many challenges unresolved, and undesirable, and achieve high the effect of improving the stability of electroless aqueous gold plating bath and sufficient plating ra
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 5 (
Inventive): Stability and Life-Time of Gold Plating Baths
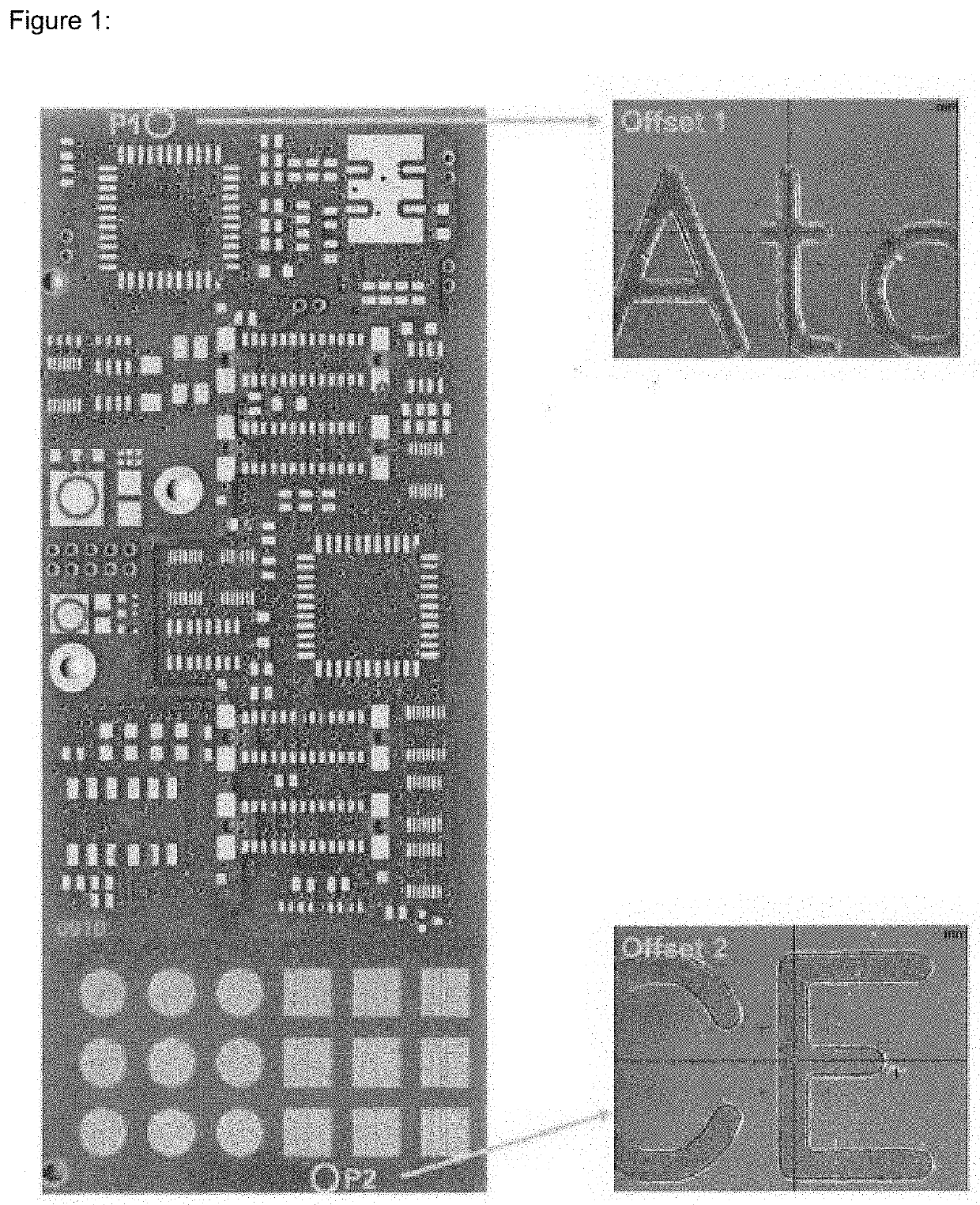
[0078]The gold plating baths of examples 1 to 3 were used to deposit gold on substrates for a prolonged time. The stability of the gold plating baths and the plating rate were monitored over time. If a plate-out occurred the solution was filtered and re-used. Every day during the experiment, the pH value was measured and adjusted to 7.1 with KOH and / or H3PO4 if necessary. During plating, the source of gold ions, the source of cyanide ions and the plating enhancer compound were continuously replenished.
[0079]Table 6 provides information on the stability of gold plating baths containing different plating enhancer compounds. The plating baths were visually inspected directly after make-up (day 0) and for one week on a daily basis. The gold plating baths were also used to deposit gold on substrates every day during this test period. These results are summarized in Table 7. The values given in said table are the deposit thickness i...
example 6 (
Inventive): Ratio of Plating Enhancer Compound to Reducing Agent for Gold Ions
[0081]A gold plating baths containing the following components was prepared by dissolving all components in water:
Water100 mLpotassium hydroxide12.4 g / LN1,N2-di-iso-propylethane-1,2-diaminesee table 8complexing agent89 mmol / Lsulphur based stabilising agent1.5 mg / Lthallium ions4.4 mg / Lpotassium cyanide 42 mg / Lformaldehyde 0.3 g / Lgold ion source1.47 g / L
[0082]The gold plating bath was adjusted with KOH / H3PO4 to a pH value of 7.1. A substrate was subjected to the process as described in table 1 wherein the electroless gold plating step was carried out for 10 min only.
[0083]The process was repeated several times with different gold plating baths containing increasing amounts of plating enhancer compounds whereby the amount of reducing agent for gold ions was kept at the same level. The results are provided by table 8.
TABLE 8Ratio of plating enhancer compound and reducing agent for gold ions.Molar ratio of N1,N2...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
| Molar density | aaaaa | aaaaa |
| Dissociation constant | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com