Treating Auto-Immune and Auto-Inflammatory Diseases
a technology for auto-immune and auto-inflammatory diseases, applied in the direction of pharmaceutical delivery mechanism, powder delivery, medical preparations, etc., can solve the problems of reduced t cell responsiveness to gcs, high risk of developing very serious side effects, and complex contextual dependence of gc signaling mechanism, so as to restore corticosteroid sensitivity and enhance glucocorticoid sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
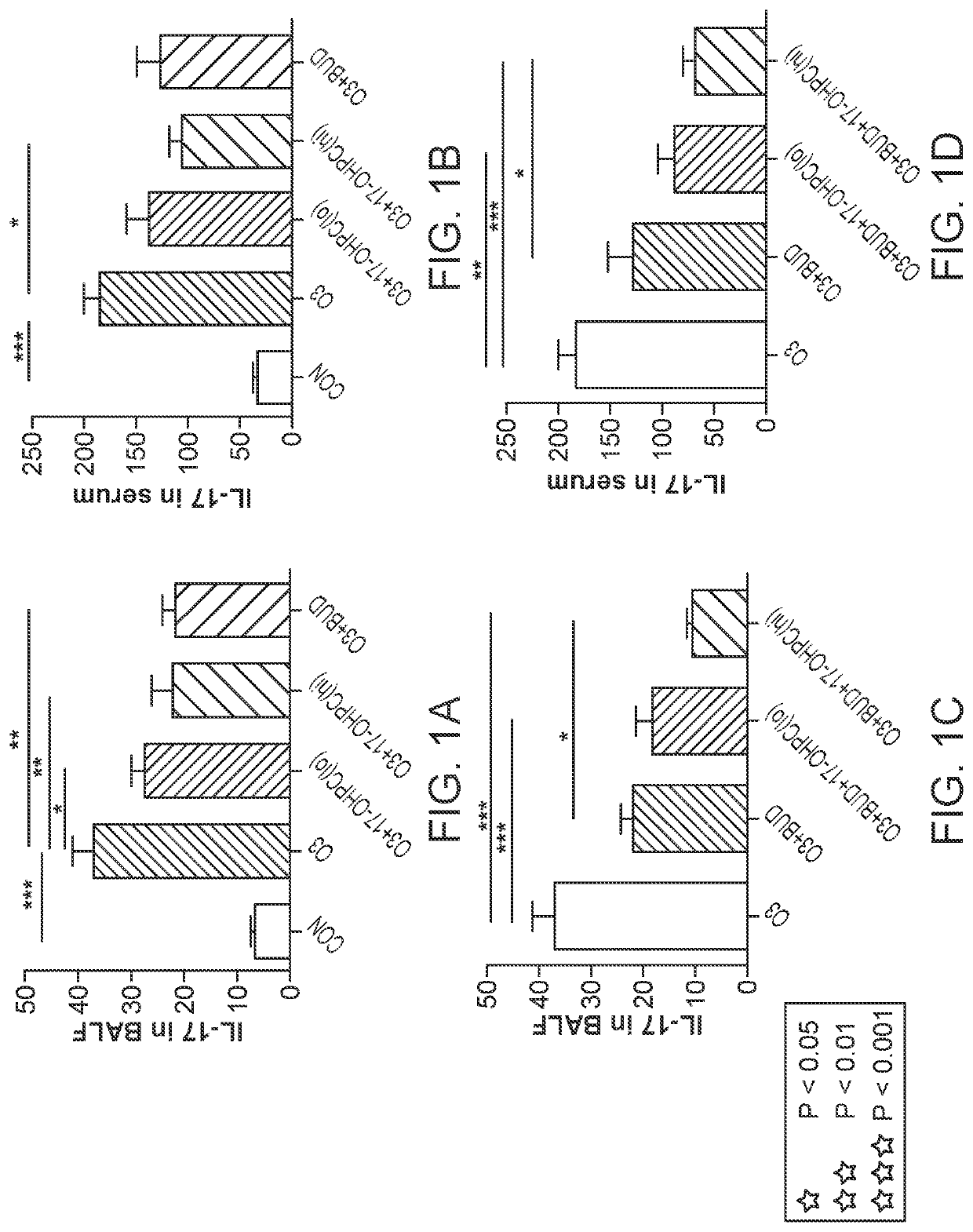
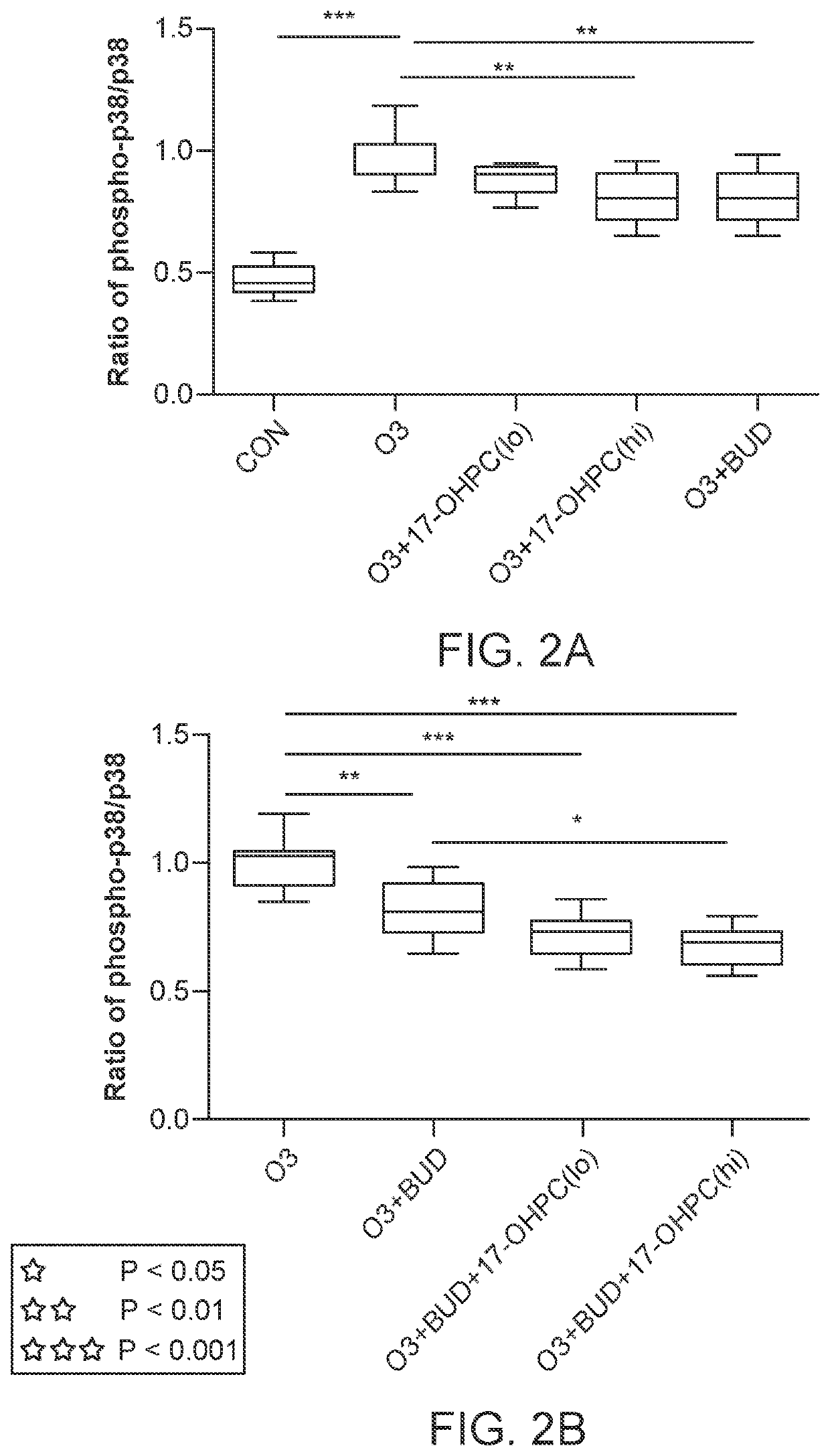
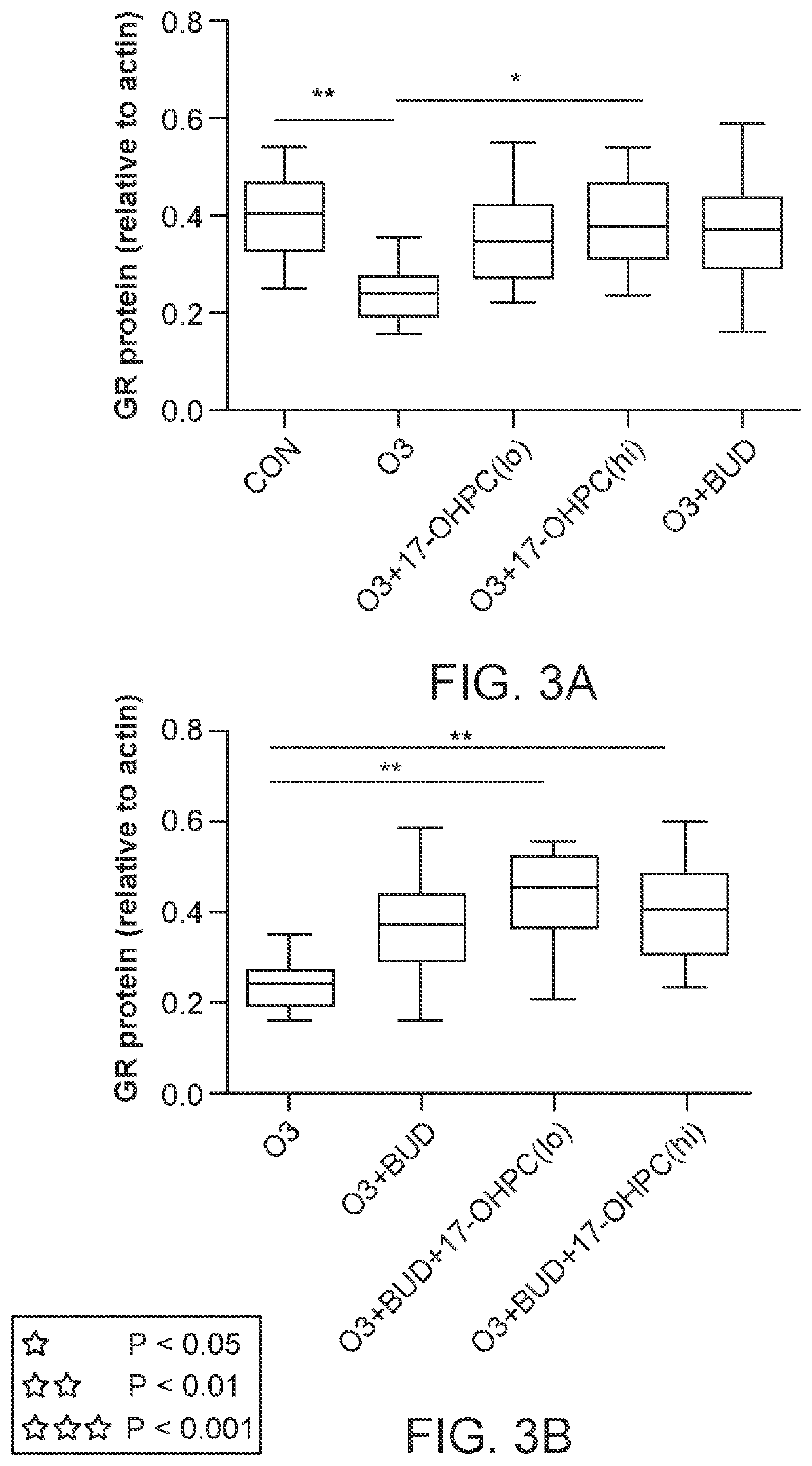
lass="d_n">[0069]According to various embodiments, a method of treatment using a pharmaceutical formulation comprising 17-OHPC has shown to be effective in two specific mechanisms: lowering IL-17 levels and inhibiting p38 MAPK activity. Both actions have a therapeutic effect in treating IL-17 and p38 MAPK mediated glucocorticoid resistance. Such a result is surprising given that previous studies using P4 had contrary results.
[0070]P4 and 17-OHPC are both classified as progestogen hormones. P4 is a naturally occurring progestogen endogenous to living organisms and may be considered as the chemical equivalent of a genus. P4 is synthesized within the ovaries, testes, placenta, and adrenal gland. Whereas 17-OHPC is a progestin (a synthetic progestogen) derived from 17α-hydroxyprogesterone (17-OHP) and caproic acid.
[0071]Structurally, progesterone (molecular weight (MW) of 314.46 g / mol and a melting point of 129.5° C.) and 17-OHPC (MW 428.6 g / mol and a melting point of 119° C.) and proge...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com