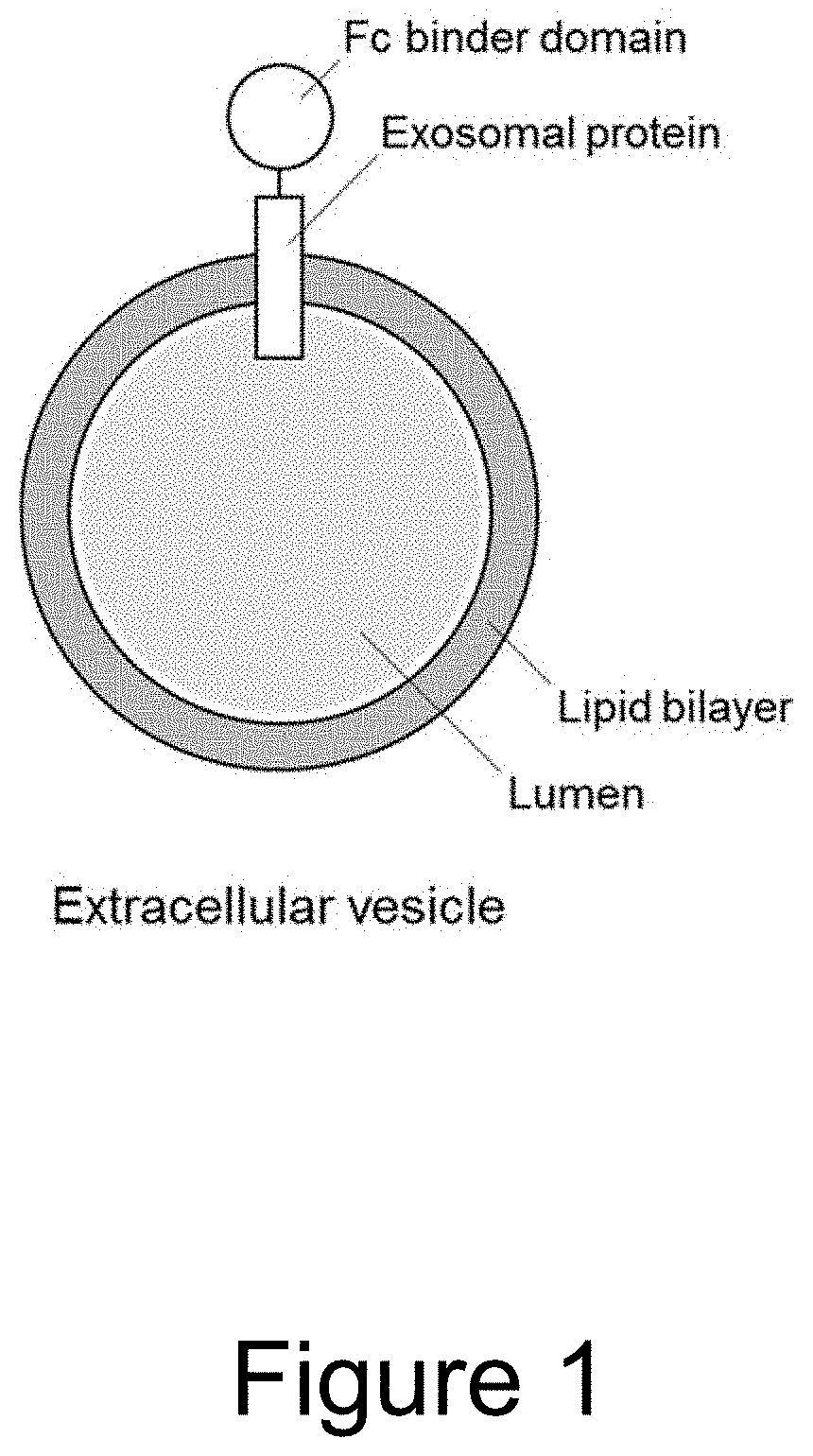
Extracellular vesicle comprising a fusion protein having fc binding capacity
a technology of fusion protein and extracellular vesicle, which is applied in the field of extracellular vesicle (ev) therapeutics, can solve the problems of increased surface coating of evs with the protein and fc domain, and achieve the effect of enhancing the therapeutic potential of evs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
f IgG to EVs Comprising Fc Binding Polypeptides (Fc-Binding EVs)
[0107]EVs were isolated from the conditioned medium from engineered HEK293T cells (control versus Fc-binding construct that stably express Gp130 Extracellular domain-2XGGGGS linker-Z domain-Gp130 transmembrane domain-Leucine Zipper-N terminal syntenin-His tag) using tangential flow filtration with 300 kd hollow fiber columns, followed by ultrafiltration using 10 kd spin filters for concentration. The binding capacity for IgG by the Fc-binding EVs were then assessed using electron microscopy and flow cytometry.
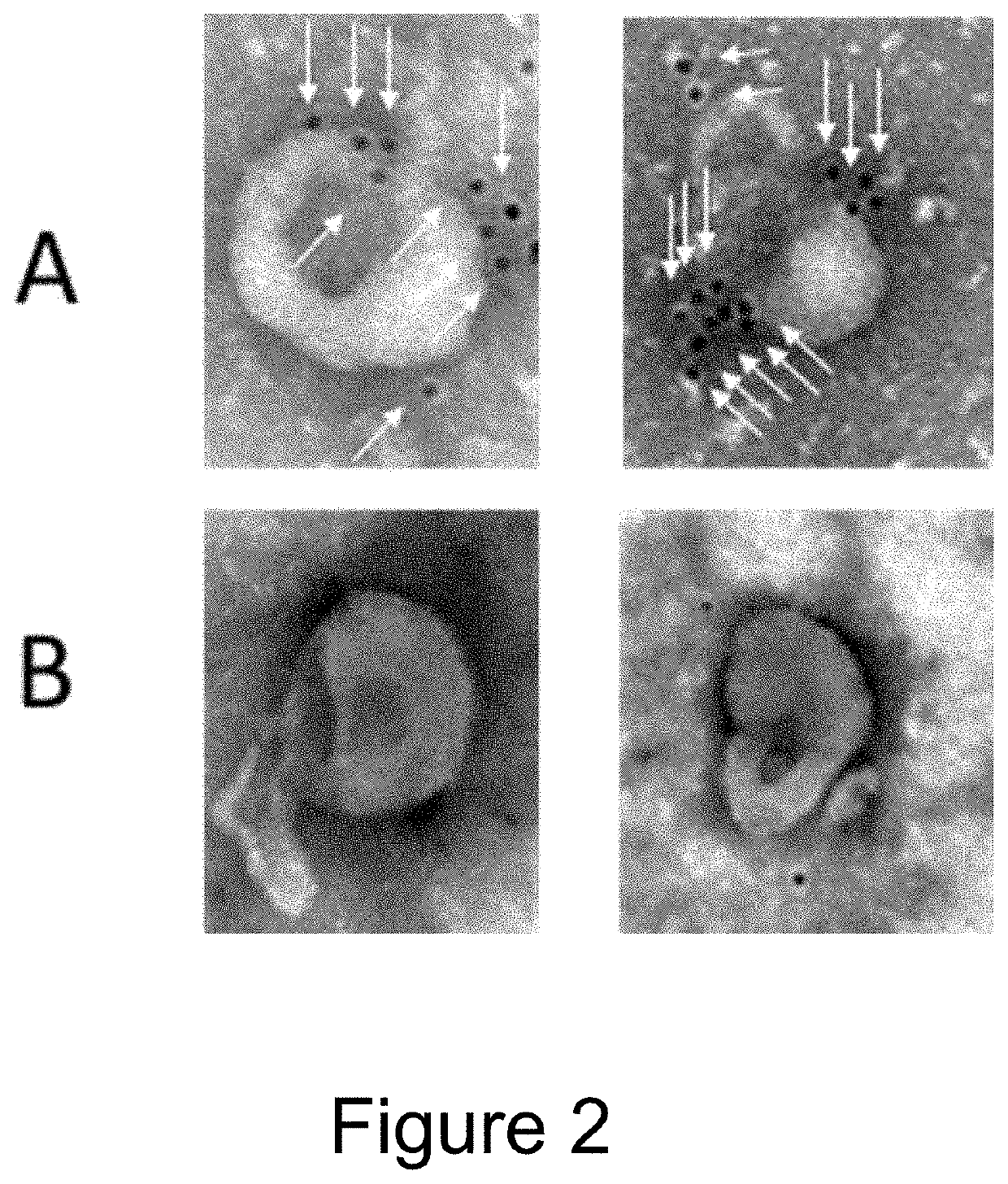
[0108]For electron microscopy, 1×10{circumflex over ( )}9 EVs were incubated with Rabbit anti-goat 10 nm antibody conjugated with gold Nanoparticles for 2 h at 37° C. As shown in FIG. 2, Fc-binding EVs (A) are decorated with nanogold labeled antibodies (i.e. Fc containing proteins), whereas control EVs (B) do not have any antibodies bound.
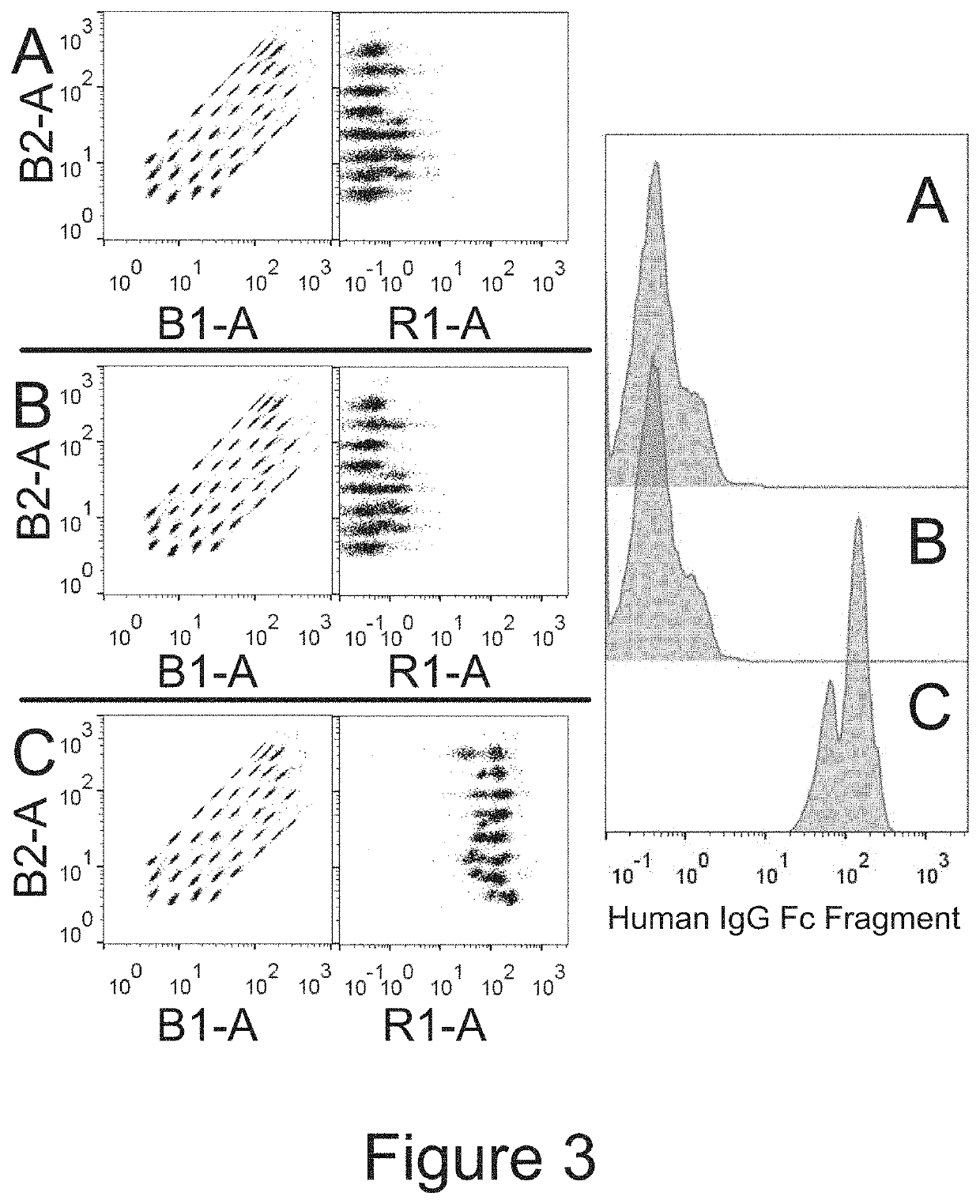
[0109]For flow cytometry, 1×10{circumflex over ( )}8 EVs were incubated overn...
example 2
mp2B EVs for Delivery of Anti-HER2 Antibody
[0110]EVs were isolated from HEK293T cells (either stably expressing FCGR1A Extracellular domain-4XGSlinker-Lamp2b or their wild type controls) using ultrafiltration and size exclusion chromatography. EVs were labelled with PKH26 red fluorescent dye, and decorated with anti-HER2 antibody or its isotype control by co-incubating EVs and antibody for 1 h at 37° C. Unbound antibody was removed by size exclusion chromatography. Uptake of antibody decorated EVs was characterized in HER2 low-expressing cell line MDA-MB-231 and in HER2 high-expressing cell line MDA-MB-361 using flow cytometry. FIG. 4 shows that anti-HER2 antibody increases uptake of decorated EVs as compared to isotype control decorated and wild type EVs only in HER2 high-expressing cell line MDA-MB-361, but not in HER2 low-expressing cell line MDA-MB-231. Similar results were obtained with EVs expressing CD63-ZZ fusion proteins.
example 3
t Delivery by EVs Comprising CD81-Protein A / G Fusion Proteins
[0111]EVs were isolated from HEK293T cells (either stably expressing CD81-ProteinA / G CD81 Second loop fusion protein or their wild type control) using ultrafiltration and size exclusion chromatography. EVs were decorated with etanercept or a control antibody by co-incubating EVs and etanercept for 1 h at 37° C. Unbound etanercept was removed by size exclusion chromatography. To study anti-inflammatory effect of the etanercept-decorated EVs, the well-studied TNBS-induced colitis mouse model was used. This model simulates the gut inflammation, cytokine storm and weight decrease associated with IBD patients. 24 mice were divided into four treatment groups, with 6 mice per group. The mice were pre-sensitized by applying 150 μl of a olive oil-acetate solution with 2% TNBS, on the skin, 1 week prior to colitis induction. Colitis was then induced by giving a rectal infusion of 100 μl solution containing 1.5% TNBS in 40% ethanol. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com