Novel Anti-hepatitis b virus antibody and uses thereof
a technology of hepatitis b virus and anti-hepatitis b virus, which is applied in the field of molecular virology and immunology, can solve the problems of low clinical efficacy, low price, and less sources of high-potency plasma
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
eening of pH-Dependent Anti-HBsAg Antibody
[0193]1.1 Determination of Mutation Site for pH-Dependent Antibody Modification
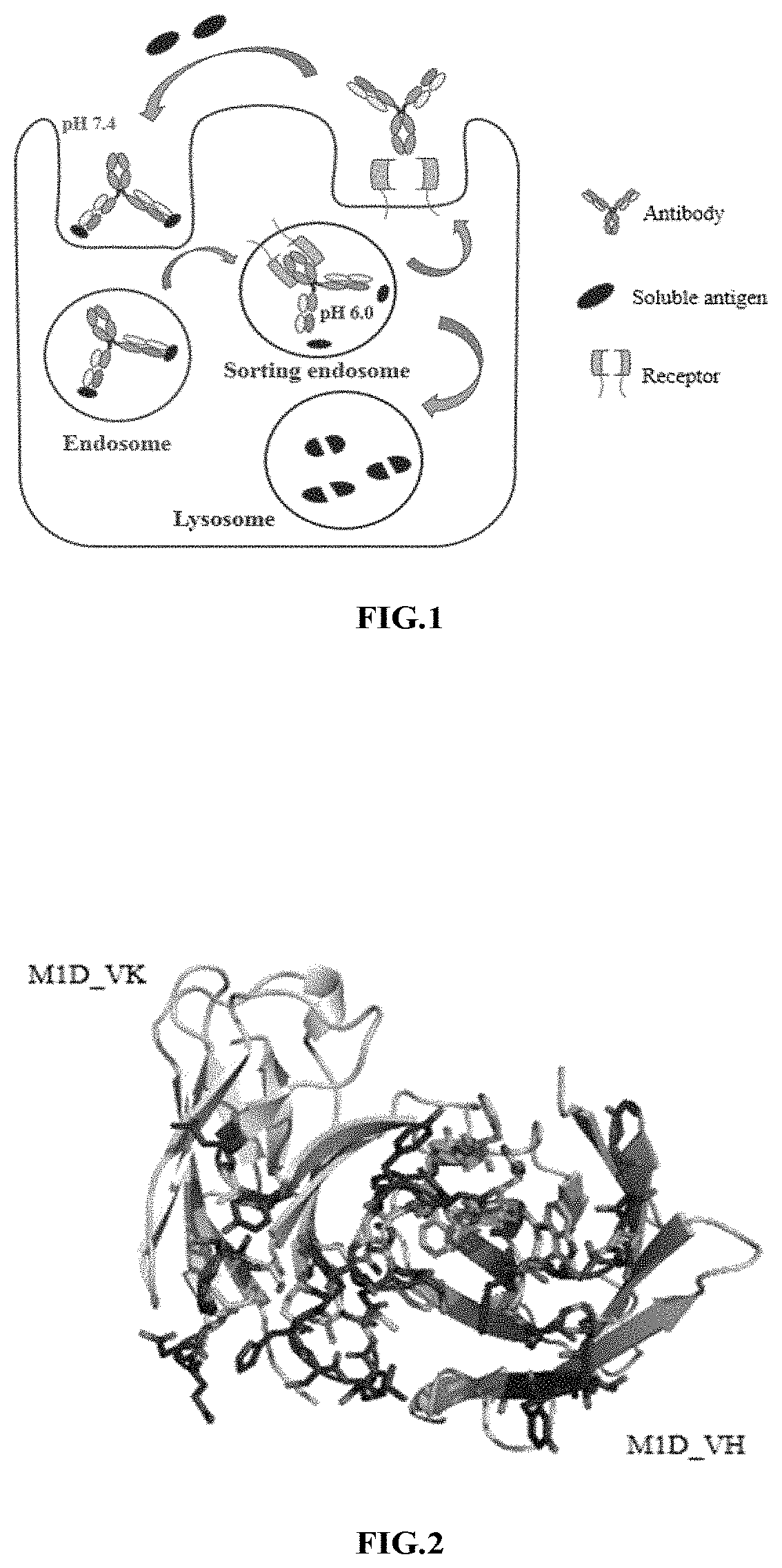
[0194]The anti-HBV humanized antibody M1D (described in detail in Chinese Patent Application 201810307136.5) previously developed in the laboratory was used as the parent antibody, its variable regions were modified for pH-dependent antigen binding. As shown in FIG. 1, the modified M1D could maintain the antigen-binding activity under neutral conditions, but had a significant decrease in antigen-binding activity under acidic conditions; the dissociated modified M1D could bind to intracellular FcRn so as to return to the plasma and bind to antigen again, so that one molecule of M1D after pH-dependent antigen binding modification could repeatedly bind to and neutralize a plurality of molecules of antigens. Histidine could be protonated under acidic conditions and was a key amino acid to bring pH-dependent antigen binding properties. The three-dimensional structure o...
example 2
on of pH-Dependent Anti-HBsAg Antibody
[0200]2.1 Construction of Recombinant Vector for Eukaryotic Expression
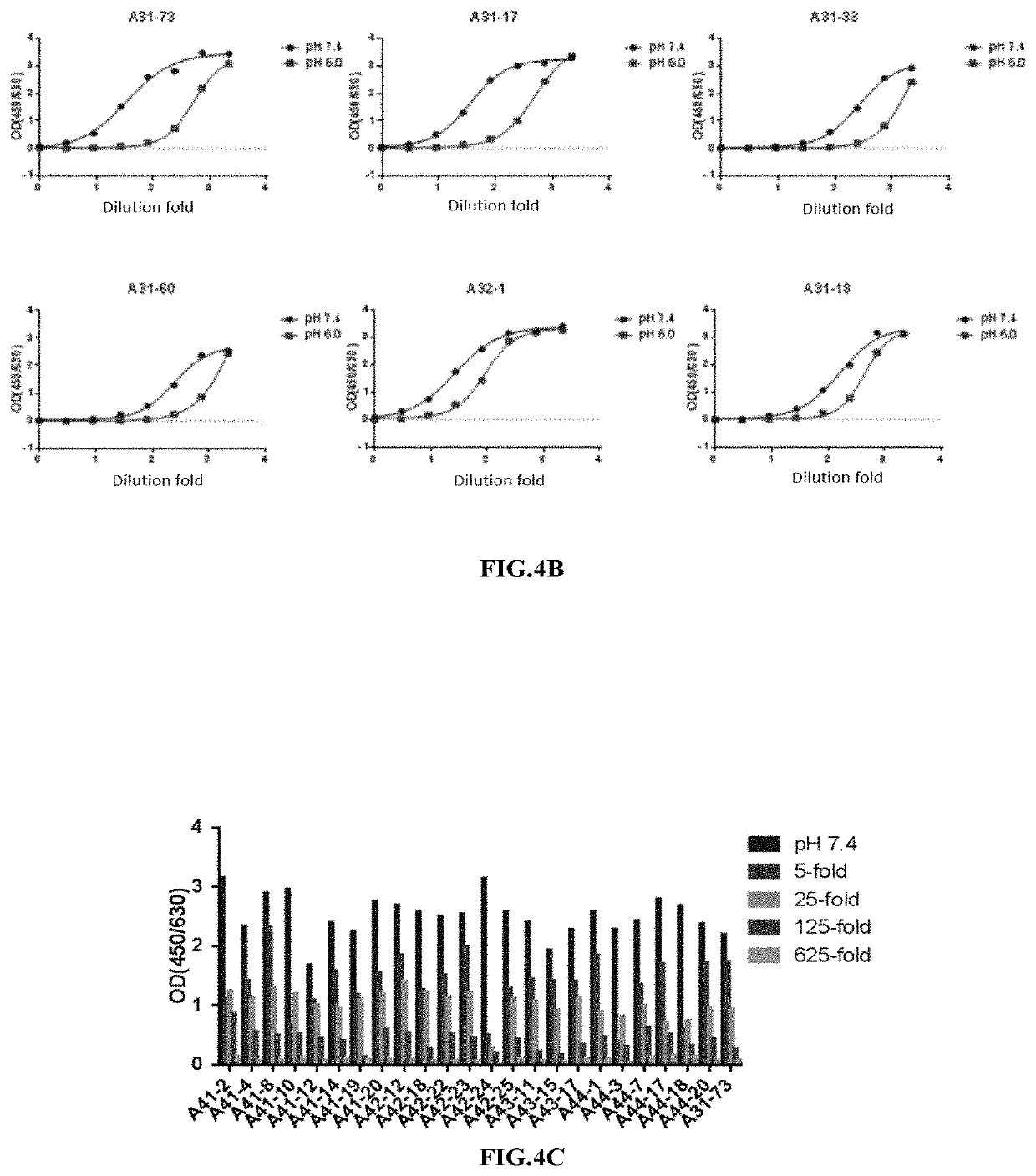
[0201]In the present invention, a large amount of antibody recombination needed to be carried out, so it was necessary to construct a set of light and heavy chain vectors that can efficiently recombine antibodies. In the present invention, the existing eukaryotic expression vector pTT5 in the laboratory was specially modified to construct a set of light and heavy chain recombinant vectors for double plasmid co-transfection. MGWSCIILFLVATATGVHS (SEQ TD NO: 49) was used as the signal peptide for the light and heavy chains. The sequences encoding the constant regions of the human antibody light and heavy chains were separately ligated to the downstream of signal peptide to construct a set of eukaryotic expression vectors pTT5-CH, pTT5-Cκ and pTT5-Cλ that facilitated antibody recombination.
[0202]The four scFv antibodies obtained in 1.3 were used to amplify the light and heavy chai...
example 3
Analysis and Functional Evaluation of pH-Dependent Anti-HBsAg Antibodies
[0208]The 4 strains of phage antibodies with property of pH-dependent binding to HBsAg that were obtained through the preliminary screening by the method of Example 1 were named as A31-73, A42-13, A42-23 and A41-8, respectively. Furthermore, the four strains of phage antibodies were subjected to the eukaryotic expression and purification by the method of Example 2. The VH and VL amino acid sequences of the four antibodies were shown in the table below. In addition, the CDR sequences of the four antibodies were determined, and the amino acid sequences of the CDRs of the heavy chain variable regions and the light chain variable regions were shown in Table 5. The mutation sites that endowed A31-73, A42-13, A42-23 and A41-8 with the property of pH-dependent antigen-binding to HBsAg were summarized in Table 5.
TABLE 4Amino acid sequences of A31-73 / A42-12 / A42-23 / A41-8 lightand heavy chain variable regionsSequenceSEQ ID...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com