Pleuromutilin (e)-4-(1-imidazoylmethyl )cinnamic acid ester with Anti-drug resistant bacteria activity and a method of preparing the same
a technology of cinnamic acid ester and pleuromutilin, which is applied in the field of medicinal chemistry, can solve problems such as huge challenges
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
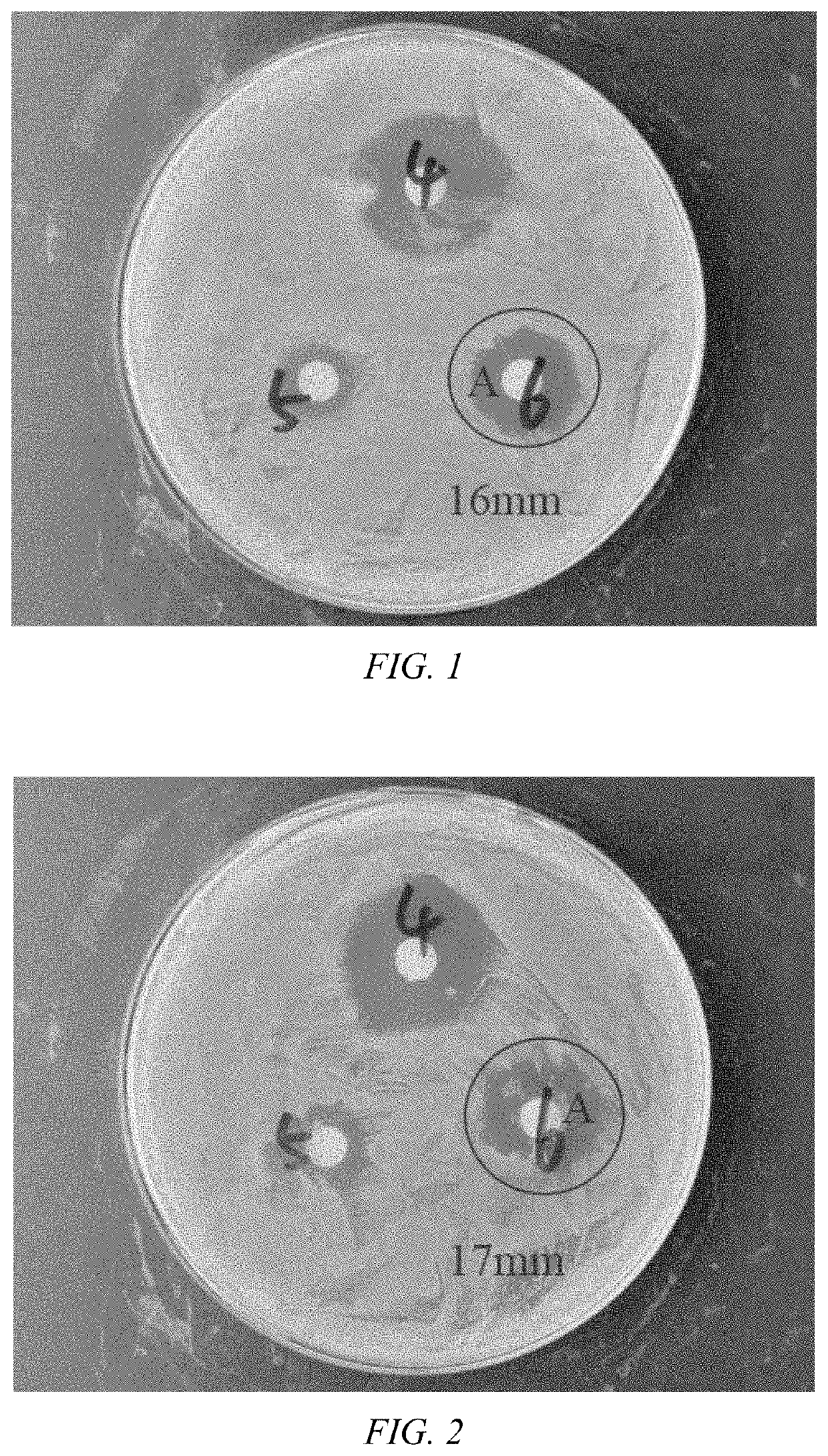
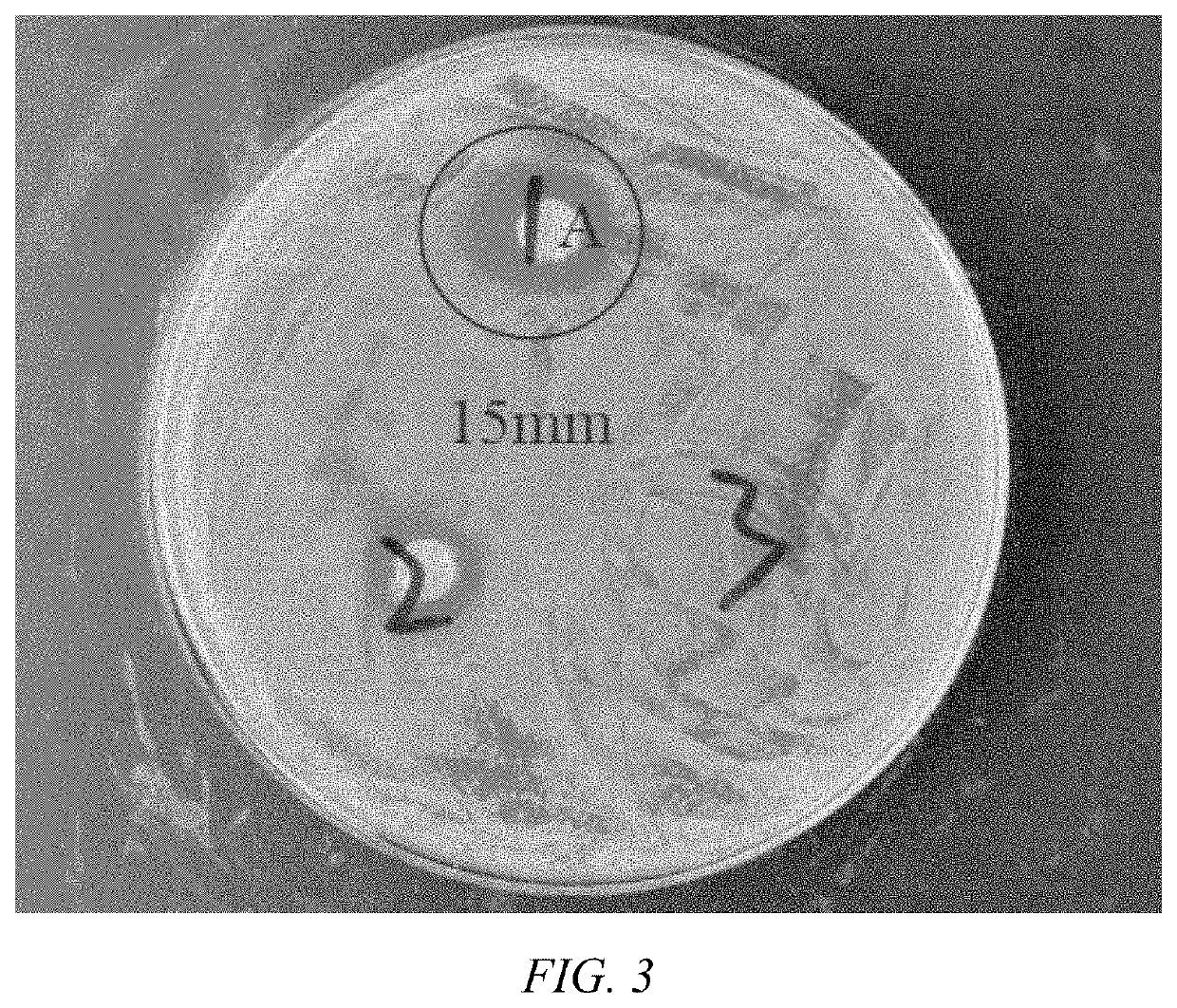
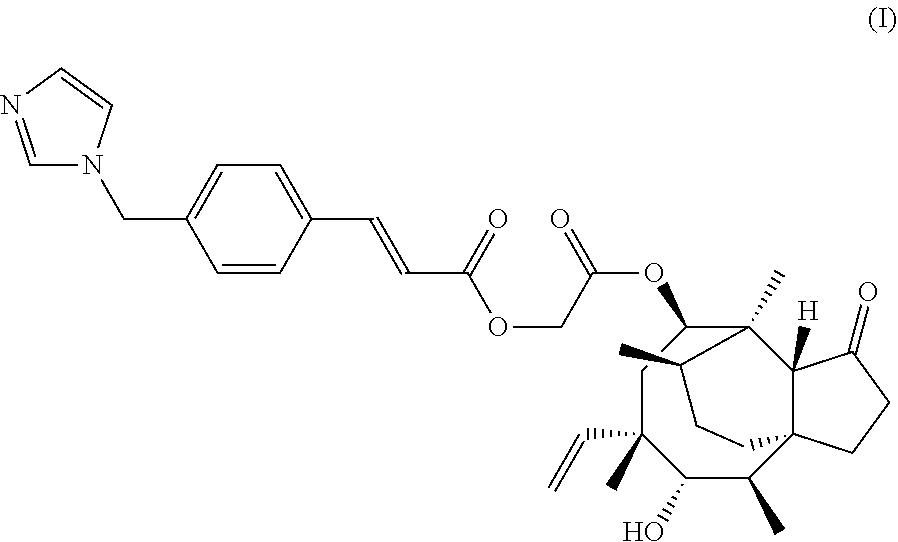
Image
Examples
example 1
Preparation of pleuromutilin (E)-4-(1-imidazoylmethyl)cinnamic acid ester (compound of formula I) ((E)-2-(((3aS,4R,5S,6S,8R,9R,9aR,12R)-5-hydroxy-4,6,9,12-tetramethyl-1-oxo-6-vinyldecahydro-3a,9-propanocyclopenta[8]annulen-8-yl)oxy)-2-oxoethyl-3-(4-((1H-imidazol-1-yl)methyl)phenyl)acrylate)
[0030]In a 100 mL three-necked flask, 246.0 mg (0.65 mmol) of pleuromutilin and 6.1 mg (0.06 mmol) of triethylamine were dissolved in 25 mL of dichloromethane under nitrogen atmosphere. 177.2 mg (0.72 mmol) of (E)-4-(1-imidazoylmethyl)cinnamic acid chloride (compound of formula III) was dissolved in 10 mL of dichloromethane, and added dropwise to the reaction mixture by a separatory funnel. After the completion of the dropwise addition, the reaction was carried out at 25° C. for 5 hours. Thin layer chromatography was used to track the reaction to completion, heating was stopped, and the protective device was removed. The reaction mixture was concentrated, washed in water, extracted with ethyl acet...
example 2
Preparation of pleuromutilin (E)-4-(1-imidazoylmethyl)cinnamic acid ester
[0032]In a 100 mL three-necked flask, 246.0 mg (0.65 mmol) of pleuromutilin and 6.1 mg (0.06 mmol) of triethylamine were dissolved in 25 mL of dichloromethane under nitrogen atmosphere. 159.9 mg (0.65 mmol) of (E)-4-(1-imidazoylmethyl)cinnamic acid chloride was dissolved in 10 mL of dichloromethane, and added dropwise to the reaction mixture by a separatory funnel. After the completion of the dropwise addition, the reaction was carried out at 25° C. for 4 hours. Thin layer chromatography was used to track the reaction to completion, heating was stopped, and the protective device was removed. The reaction mixture was concentrated, washed in water, extracted with ethyl acetate, concentrated and dried to obtain a crude product. The crude product was purified by silica gel column chromatography with dichloromethane:methanol=8:1 as eluent, and the eluent was combined and concentrated under reduced pressure to obtain...
example 3
Preparation of pleuromutilin (E)-4-(1-imidazoylmethyl)cinnamic acid ester
[0033]In a 100 mL three-necked flask, 246.0 mg (0.65 mmol) of pleuromutilin and 6.1 mg (0.06 mmol) of triethylamine were dissolved in 25 mL of dichloromethane under nitrogen atmosphere. 191.9 mg (0.78 mmol) of (E)-4-(1-imidazoylmethyl)cinnamic acid chloride was dissolved in 10 mL of dichloromethane, and added dropwise to the reaction mixture by a separatory funnel. After the completion of the dropwise addition, the reaction was carried out at 25° C. for 5 hours. Thin layer chromatography was used to track the reaction to completion, heating was stopped, and the protective device was removed. The reaction mixture was concentrated, washed in water, extracted with ethyl acetate, concentrated and dried to obtain a crude product. The crude product was purified by silica gel column chromatography with dichloromethane:methanol=10:1 as eluent, and the eluent was combined and concentrated under reduced pressure to obtai...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com