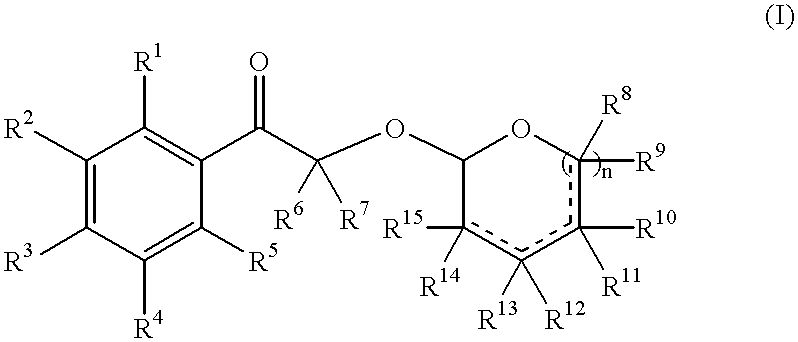
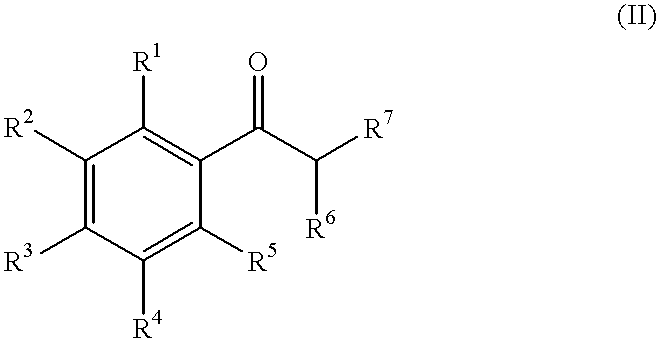
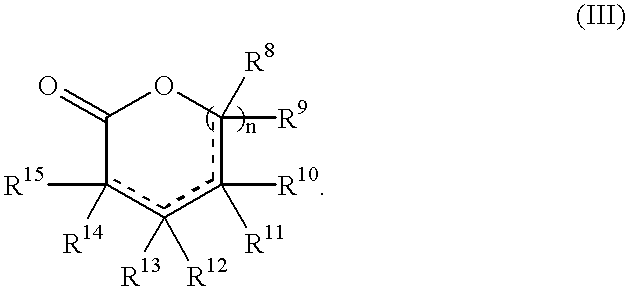
Fragrance precursors
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of Cyclic Phenacyl Acetals
1. General Procedure for the Preparation of Hydroxy-acetophenones
[0124]A suspension of the corresponding bromo-acetophenone (0.05 mmol) and sodium formate (17 g, 0.25 mol, 5 eq.) in aqueous ethanol (85%, 150 ml) was heated at reflux until completion of the reaction (TLC). Most of the ethanol was evaporated and the mixture partitioned between MTBE (80 ml) and water (70 ml). The organic phase was separated and washed with aqueous NaHCO3 (sat.) and brine. Removal of the solvent in vacuo, after drying over MgSO4, afforded a crude product as a solid which was recrystallised from ethanol.
2-Hydroxy-1-(4-methoxy-phenyl)-ethanone
[0125]Obtained according to the general procedure.
[0126]mp 104-105° C. 1H-NMR (400 MHz, CDCl3): 3.48 (t, 1H, J 4); 4.82 (d, 2H, J 4); 6.95-7.0 (m, 2H); 7.85-7.95 (m, 2H). IR (νmax, cm−1, neat): 3415m, 2929w, 1672s, 1603s. MS [m / z (EI)]: 166 (M+, 4), 155 (100), 77 (28).
1-(3,5,5,6,8,8-Hexamethyl-5′,6′,7′,8′-tetrahydro-naphthalen-2-...
example 2
Photolysis of Cyclic Phenacyl Acetals (I) in Solutions
[0164]Photorelease assays were conducted on solutions (typical concentrations of precursors (I) were from 0.05% to 0.1% g / v) in organic solvents (preferably ethanol) or on cotton towels after deposition of the phenacyl acetals (I), as described below in the example 3.
[0165]The solutions were irradiated with a mercury lamp (150 W) in a borosilicate glass apparatus (PYREX®) so as to limit the irradiation window to mainly the UVA and UVB spectrum of sun light. The alcoholic solution was irradiated for one hour and samples taken every 15 min to analyze the extent of the photolysis.
Analysis
[0166]The presence of the aryl ketone (II) and lactone (III) after photolysis in solutions was determined by using GC retention times. Samples (0.2 μl) were injected (on column injection) without further dilution. Gas chromatography-flame ionization detection (GC-FID) was carried out with a Fisons-GC 8000series apparatus, using a J&W Scientific DB-5...
example 3
Spray Tests
[0169]1 g of an approximately 0.2% cyclic phenacyl acetal (I) solution in ethanol was evenly sprayed on a Terry towel (white cotton towel, 25cm×25 cm, 45 g), corresponding to 45-75 μg / g cotton. The sprayed towels were allowed to dry in a dark and odorless place. When dry, the towels were irradiated for a few seconds up to a few minutes with a tanning lamp (Osram ULTRA-VITALUX®, 300 W; at a distance of 50 cm, the light has approximately six to seven times the effect of the natural sunlight at noon on a sea-side mid-summer day). The evaluation was done by a trained panel of perfumers before and after irradiation. Before irradiation, the towels were judged to be odorless. The results after irradiation are summarized in table 2.
[0170]
TABLE 2Release of aryl ketones and lactones from cyclicphenacyl acetals on fabric upon irradiation with a tanninglamp.Fragrance Target(perception)*Globalaryl ketoneappre-STRUCTURE(II)lactone (III)ciation*1FIXOLIDE ® (++)METHYL LAITONE ® (+)+2FIXO...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Composition | aaaaa | aaaaa |
| Exposure limit | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com