Bone grafting material, method and implant
a bone grafting and bone technology, applied in the field of bone grafting materials, can solve the problems of troublesome procedures, additional pain for patients, and undamaged bone tissue must be taken up from an unspoiled portion, so as to enhance bone formation and accelerate the formation of new bone or cartilage tissue, the effect of reducing the risk of side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0030]Two batches of bioactive glasses with compositions shown in Table 1 were first formed by melting the raw-materials in Pt-Au crucible for 2 to 3 hours at approximately 1360° C.
[0031]
TABLE 1The composition of the bioactive glasses [wt-%].GlassNa2OK2OMgOCaOB2O3P2O5SiO2A18 90141454B12155111254
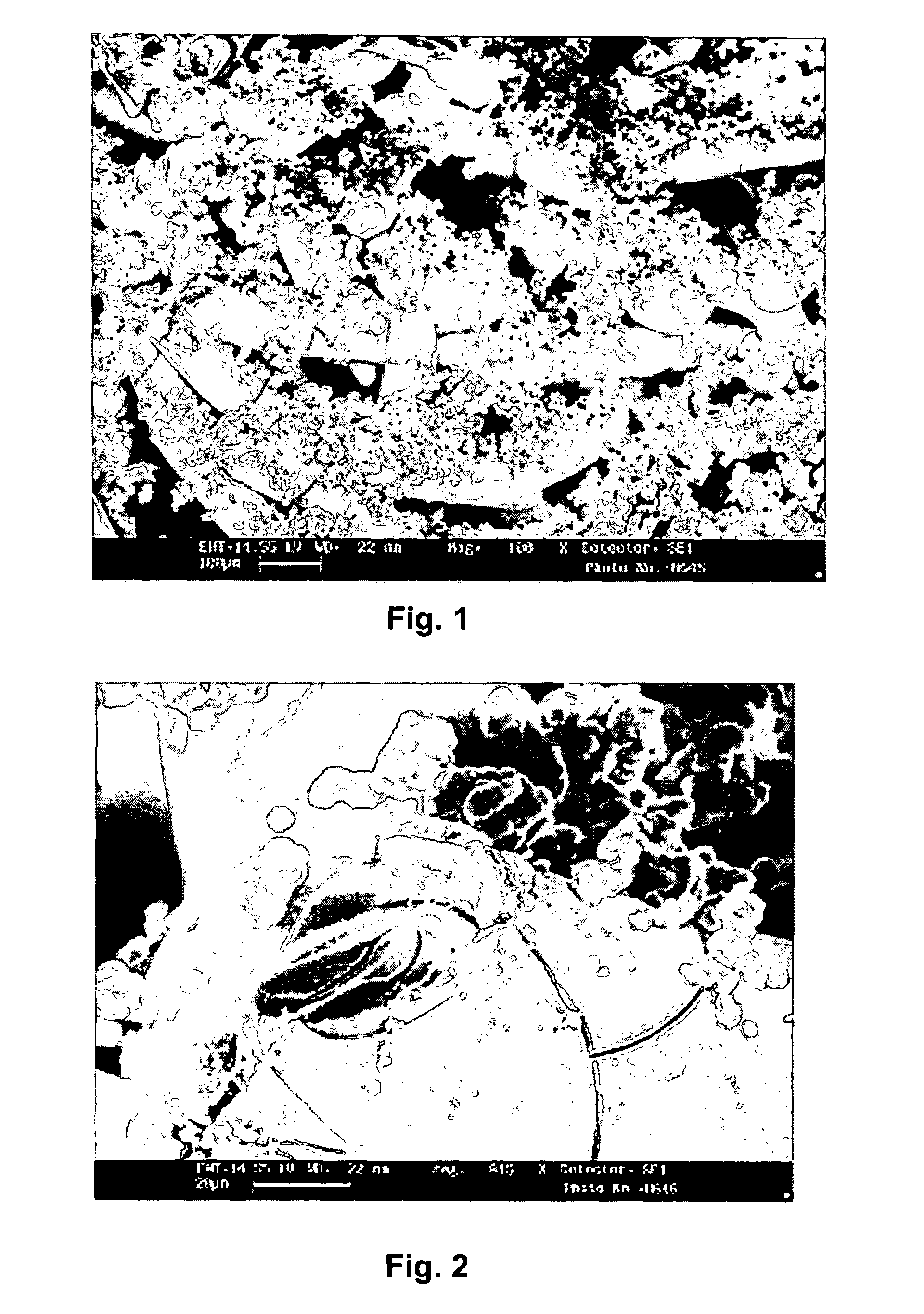
[0032]From both batches of the bioactive glasses, fibers with a diameter of approximately 75 μm were then formed by melt spinning. The fibers were further cut to have a segment length of approximately 3 mm. From the obtained fibre segments were formed 3-dimensional scaffolds with open network. The forming took place by first placing the fibre segments in a mould and then sintering the fibers at elevated temperatures.
[0033]The obtained scaffolds were then immersed into a simulated body fluid for a one-week period in order to obtain a formation of Si-rich layer and calcium phosphate (CaP) precipitation on the surface of the scaffolds. Scaffolds containing a CaP surface as a carrier layer were t...
example 2
[0048]Six pieces of bone grafting material comprising a porous bioactive glass scaffold “A” or “B” (the compositions being the same as in Example 1) and with a CaP layer on top of the glass surface were manufactured as described in example 1. All the samples were weighed with a top balance and the samples were then placed into a high-pressure chamber (with a total volume of 0.37 dl) together with 2 ml of NMP. The samples were placed on a metallic sample holder. The high-pressure chamber was closed and the chamber was first filled with a CO2 vapor at room temperature. The chamber was then heated to a temperature of 60° C. so that the pressure inside the chamber increased up to 120 bar. These conditions were remained for 24 hours, and the pressure was lowered slowly within 15 minutes to reach a normal air pressure. The weight of the samples was then measured.
[0049]
TABLE 3The amount of NMP in scaffold / carrier structures.Weight afterSample No.Initial weight [g]treatment [g]Weight-% of N...
example 3
[0050]Various bioceramics and one bioceramic composite were acquired, namely[0051]1) Synthetic calcium phosphate (CaP), 3-dimensional scaffold manufactured by sintering CaP powder,[0052]2) Bio-Oss®, an anorganic mineral bone matrix of bovine origin, and[0053]3) Hydroxyapatite (HA) powder with a particle size of 4 μm.
[0054]The samples were dried in a vacuum and further placed in a chamber containing 1-methyl-2-pyrrolidone (NMP) as described in Example 1. The masses of the samples were monitored prior to placing them in a chamber and 5 and 7 days after the placement in the chamber. The amount of absorbed NMP as weight-% is shown in Table 4.
[0055]
TABLE 4The absorption of NMP.NMP in structureNMP in structureafter 5 daysafter 7 daysSample No.[wt-%][wt-%]10.691.06232.2439.2030.160.23
[0056]Table 4 shows that the absorbtion of NMP varies significantly depending on the composition and structure of the bioceramic material. The highest amount of NMP was absorbed by Bio-Oss, approximately 40 wt...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
| total volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com