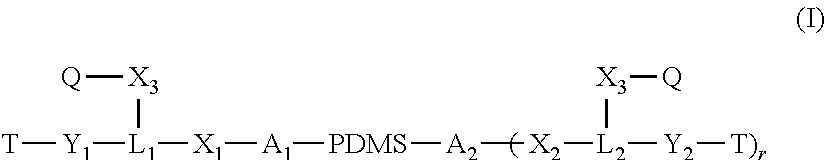
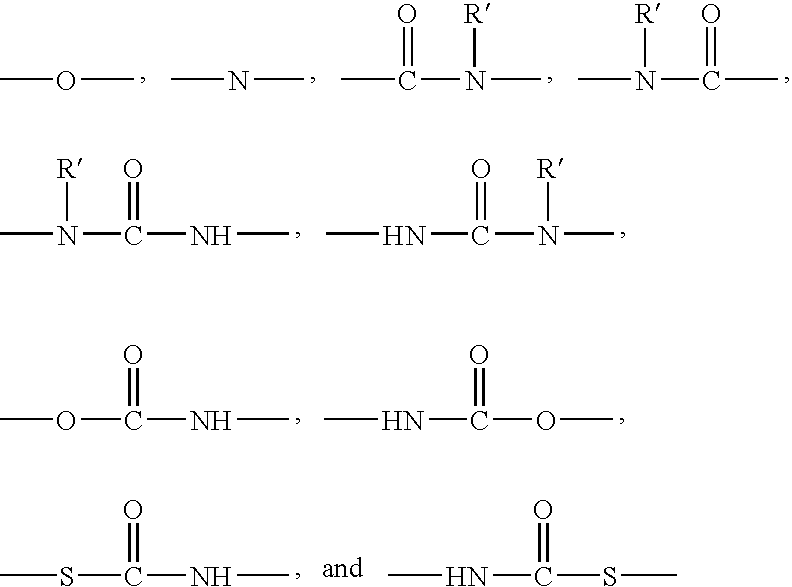
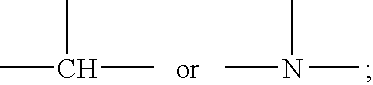Polysiloxane copolymers with terminal hydrophilic polymer chains
a technology of polysiloxane and polymer chain, which is applied in the direction of instruments, optical elements, optics, etc., can solve the problems of corneal swelling, inability to easily circumvent oxygen, and undesirable growth of blood vessels in the cornea
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Surface Hydrophilicity (Wettability) Tests
[0167]Water contact angle on a contact lens is a general measure of the surface hydrophilicity (or wettability) of the contact lens. In particular, a low water contact angle corresponds to more hydrophilic surface. Average contact angles (advancing) of contact lenses are measured using sessile drop method.
[0168]Oxygen permeability measurements. The oxygen permeability of a lens and oxygen transmissibility of a lens material is determined according to a technique similar to the one described in U.S. Pat. No. 5,760,100 and in an article by Winterton et al., (The Cornea: Transactions of the World Congress on the Cornea 111, H. D. Cavanagh Ed., Raven Press: New York 1988, pp 273-280), both of which are herein incorporated by reference in their entireties. Oxygen fluxes (J) are measured at 34° C. in a wet cell (i.e., gas streams are maintained at about 100% relative humidity) using a Dk1000 instrument (available from Applied Design and Developmen...
example 2
Reaction of Epoxy Terminated PDMS with 2-Hydroxy-2-methyl-1-phenyl-propanone
[0177]2-Hydroxy-2-methyl-1-phenyl-propanone (Daracure 1173, from CIBA) (19.8791 grams) is mixed with 20.0231 grams of epoxy terminated polydimethylsiloxane (PDMS) (DMS-E09 from Gelest), 40 mL of methylene chloride and 0.1530 grams of borontrifluoride tetrahydrofuran complex. The temperature of the reaction mixture is increased from about 22° C. to about 33° C. after the reaction components are mixed. After about 30 minutes the temperature of the reaction mixture is decreased to about 27° C. After about 6 hours at room temperature about 10 mL of methanol is added into the reaction mixture and stirred continuously for several more minutes. The reaction mixture is diluted with about 100 mL of methylene chloride and extracted with 10% sodium carbonate solution (3×50 mL), extracted with water 2×50 mL and then dried over MgSO4. The PDMS-macroinitiator is separated from MgSO4 and methylene chloride is removed by a ...
example 3
Reaction of PDMS Macro-Initiator with DMA: Preparation of Poly(DMA)-PDMS-Poly(DMA)
[0178]About 5.02 grams of the PDMS-macroinitiator from Example 2 is combined with about 30.06 grams of N,N-dimethylacrylamide (DMA) and 100 mL of ethyl acetate in a plastic beaker. A stir bar is added to the mixture and the beaker is sealed with a polyethylene bag. The mixture is stirred while being irradiated with UVA light at an intensity of about 3.5 mW / cm2. After being irradiated for a total of about 6 hours, the reaction mixture is inhibited with about 13 mg of 4-hydroxy-TEMPO dissolved in about 5 mL of ethyl acetate. The mass of the reaction mixture is adjusted to about 160 grams through the addition of ethyl acetate.
PUM
| Property | Measurement | Unit |
|---|---|---|
| water contact angle | aaaaa | aaaaa |
| elastic modulus | aaaaa | aaaaa |
| Ionoflux Diffusion Coefficient | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com