Implantable cerebrospinal fluid flow device and method of controlling flow of cerebrospinal fluid
a technology of cerebrospinal fluid and flow control, which is applied in the field of implantable cerebrospinal fluid flow control devices and methods, can solve the problems of slit ventricles, slit ventricles, neurological damage, etc., and achieve the effect of reducing the effect of intracranial pressure pulsation, reducing the resistance to flow, and improving the expected function of shunts
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0020]The entire content of U.S. Patent Application Ser. No. 60 / 741,118, filed Dec. 1, 2005, is hereby incorporated by reference.
[0021]Consistent and reliable drainage of cerebrospinal fluid from one area of the body to another, e.g., from a ventricle or ventricles of the brain to another region of the body such as the peritoneum or sagittal sinus, can be desirable. A consistent and reliable drainage method and system can minimize the expense as well as trauma and inconvenience to the patient associated with cerebrospinal fluid shunt revision surgery and can also lessen risk to the patient due to an inoperative cerebrospinal fluid drainage system.
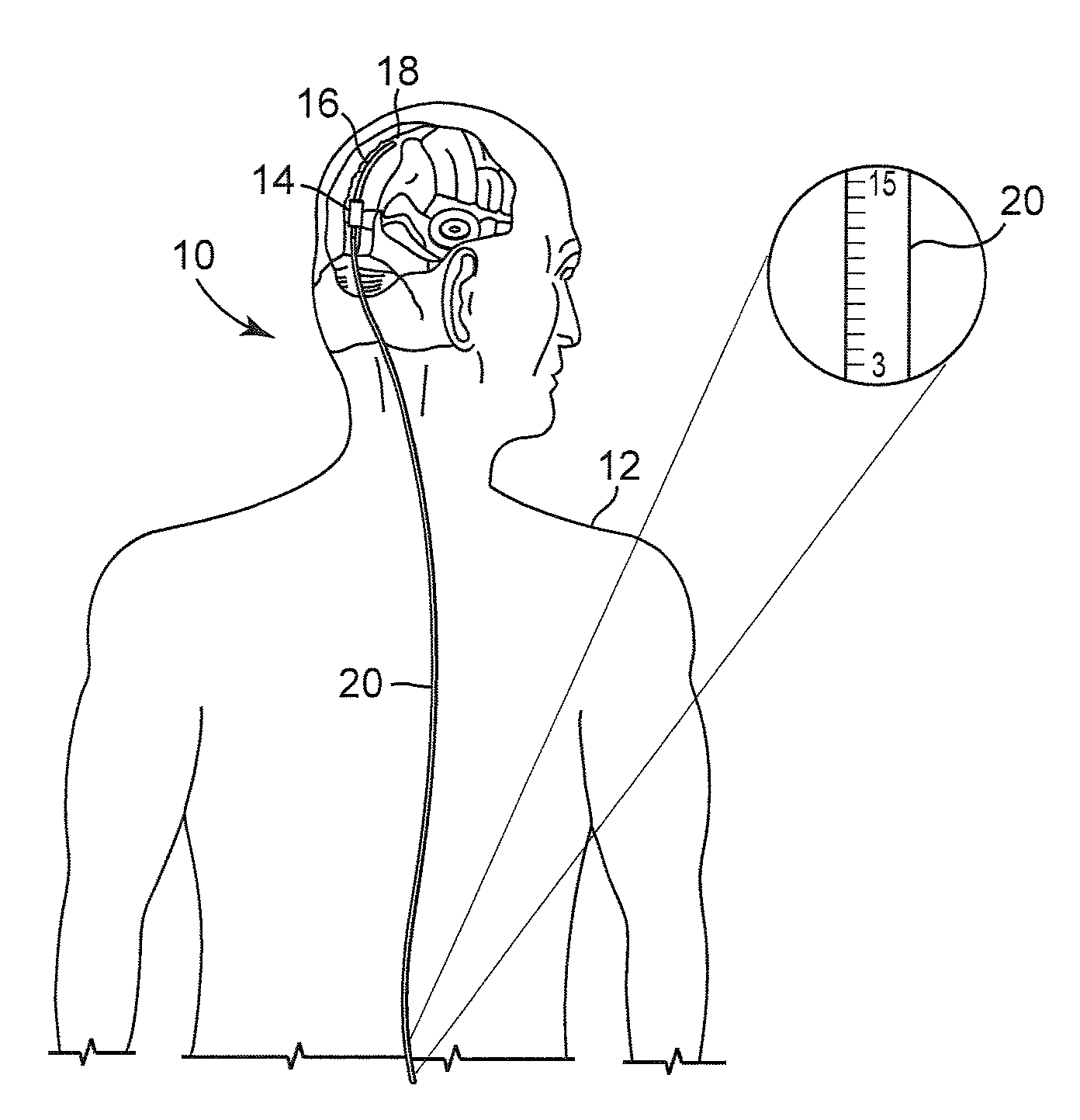
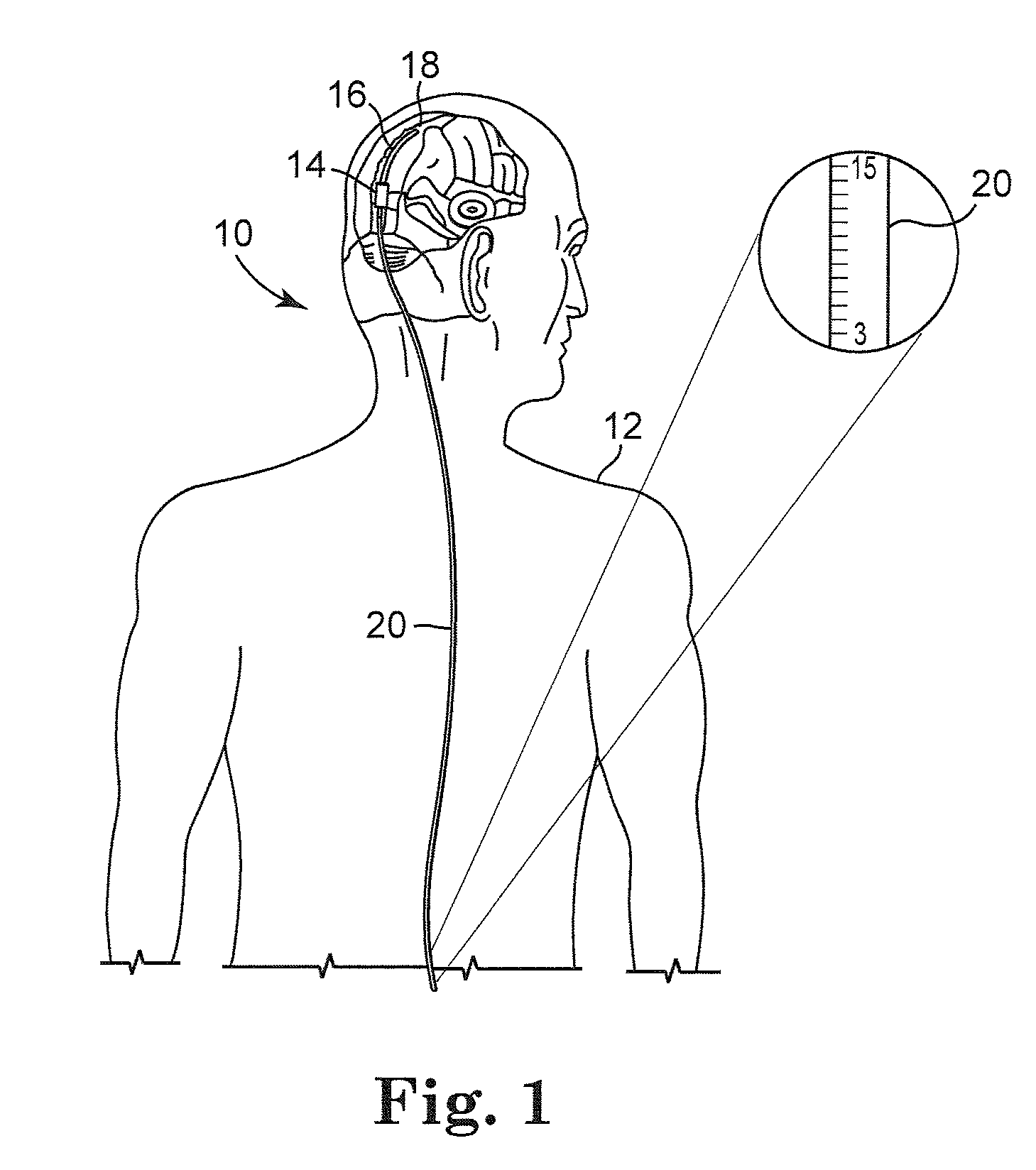
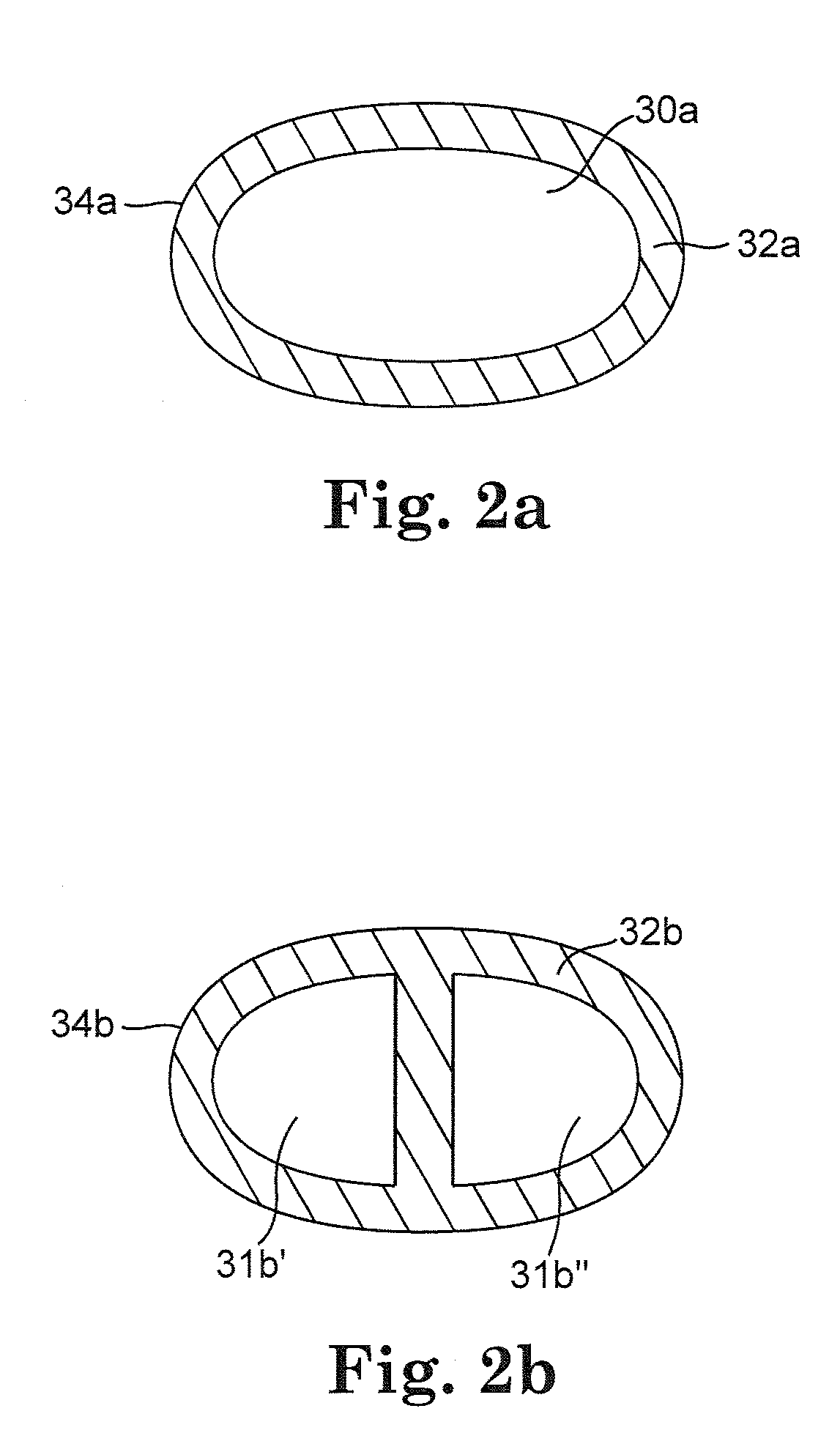
[0022]FIG. 1 illustrates an embodiment of a cerebrospinal fluid shunt 10 for draining cerebrospinal fluid from one area, for example the ventricles of the brain, of the body of patient 12 to another area of the body of patient 12. In one embodiment cerebrospinal fluid can be drained to the peritoneum, the right atrium, the sagittal sinus, o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com