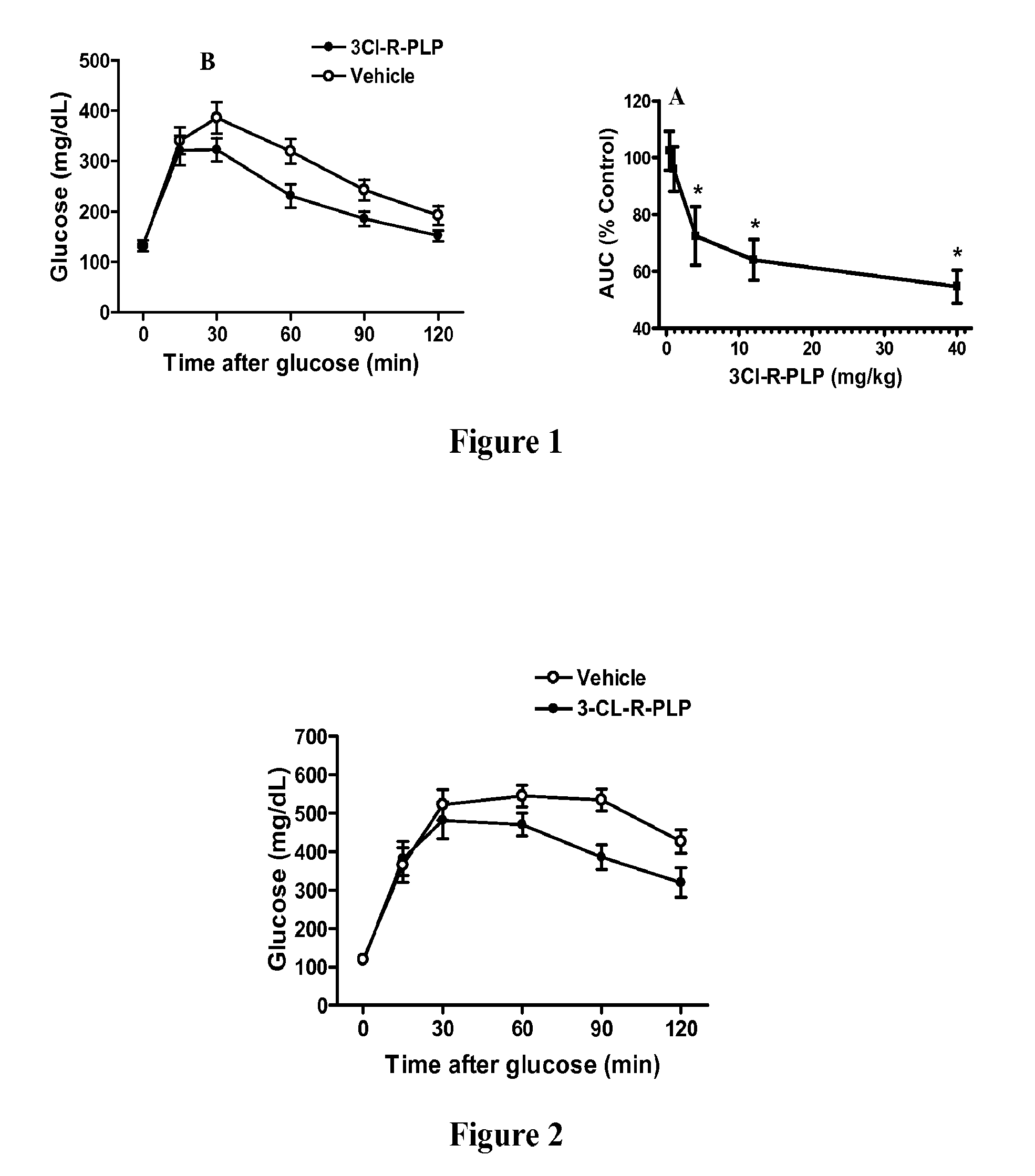
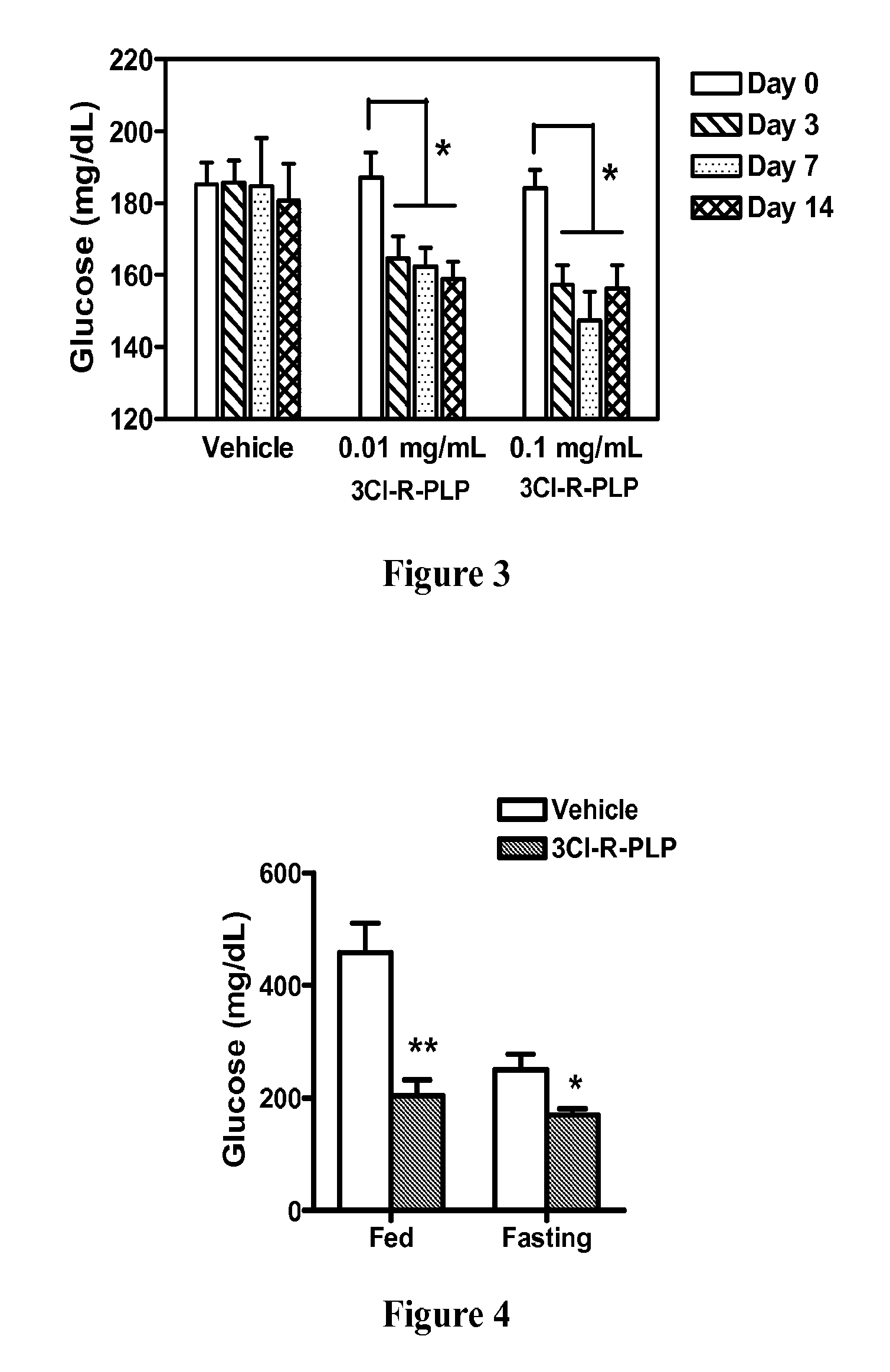
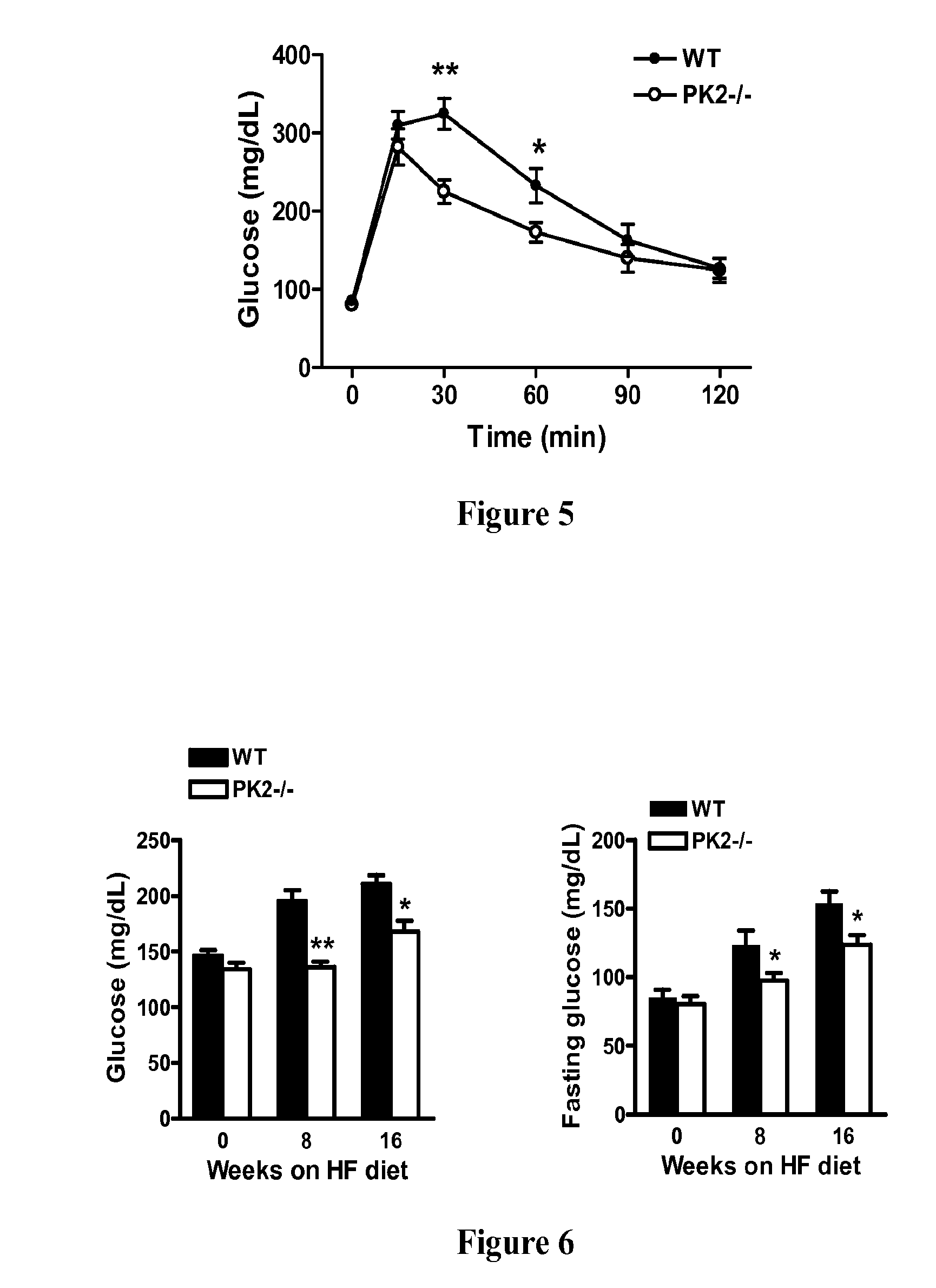
Prokineticin receptor antagonists and uses thereof
a technology of prokineticin and receptor, applied in the field of prokineticin receptor antagonists, can solve the problems of increased depression-like behaviors, impaired locomotor activity, body temperature and food intake,
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0094]
(+)-(2S)-2-Amino-N-(9-chloro-3,4-dihydro-2H-1,5-benzodioxepin-7-ylmethyl)-N-isobutylpropanamide
[0095]Step 1: 3-chloro-4,5-dihydroxy benzaldehyde: A solution of 19.1 g 3-chloro-4-hydroxy-5-methoxy benzaldehyde in dichloromethane (1600 ml) was cooled in ice water bath. Boron tribromide (53.8 g) in dichloromethane (80 ml) were added and the mixture was stirred for two hours at ambient temperature and then was concentrated. The residue was cooled again with ice water bath and precipitated with ice-cold aqueous hydrochloric acid (1N, 500 ml). Solid residue was received upon filtration, then washed with ice water (500 ml) and dried in the air to obtain 19.3 g crude product of 3-chloro-4,5-dihydroxy benzaldehyde, which was used for the following step.
[0096]Step 2: 9-chloro-3,4-dihydro-2H-1,5-benzodioxepine-7-carbaldehyde: A mixture of 3-chloro-4,5-dihydroxy benzaldehyde (4.15 g), of 1,3-dibromopropane (4.71 g, 0.92 eq.) and potassium carbonate (8.28 g, 2.5 eq.) in acetonitrile (160 m...
example 2
[0100]
(±)-2-Methyl-3-(benzylamino)-N-(9-chloro-3,4-dihydro-2H-1,5-benzodioxepin-7-ylmethyl)-N-isobutylpropanamide
[0101]Step 1: (±)-2-Methyl-3-[(2-methylpropan-2-yl)oxycarbonylamino]propanoic acid: A mixture of (±)-2-Methyl-3-aminopropanoic acid (700 mg), di-tert-butyl pyrocarbonate (2.219 g), and triethylamine (1.375 g) in DCM (200 ml) was stirred over night in room temperature. The solvent was evaporated in vac and the solid residue was used for the next step.
[0102]Step 2: (±)-2-Methyl-3-(methylpropan-2-yl)oxycarbonylamino-N-(9-chloro-3,4-dihydro-2H-1,5-benzodioxepin-7-ylmethyl)-N-isobutylpropanamide: The mixture of N-(9-chloro-3,4-dihydro-2H-1,5-benzodioxepin-7-ylmethyl)-2-methylpropan-1-amine (600 mg), (±)-2-Methyl-3-[(2-methylpropan-2-yl)oxycarbonylamino]propanoic acid (200 mg), 1-ethyl-3-(3-dimethylaminopropyl)-carbodiimide hydrochloride (475 mg) and of dimethyl aminopyridine (284 mg) in DCM (30 ml) was stirred overnight. The reaction mixture was partitioned between ethyl aceta...
example 3
[0105]
(±)-2-Methyl-3-(benzyl(methyl)amino)-N-(9-chloro-3,4-dihydro-2H-1,5-benzodioxepin-7-ylmethyl)-N-isobutylpropanamide
[0106]Step 1: (±)-2-Methyl-3-(benzyl(methyl)amino)-N-(9-chloro-3,4-dihydro-2H-1,5-benzodioxepin-7-ylmethyl)-N-isobutylpropanamide: A mixture of (±)-2-Methyl-3-(benzylamino)-N-(9-chloro-3,4-dihydro-2H-1,5-benzodioxepin-7-ylmethyl)-N-isobutylpropanamide (26 mg), paraformaldehye (16 mg), glacial acetic acid (90 μl) and sodium triacetoxyborohydride (26 mg) was dissolved in tetrahydrofuran (5 ml), and stirred overnight. The mixture was partitioned between aq. potassium carbonate (1N, 10 mL) and ethyl acetate (15 ml). The organic layer was concentrated and the residue was purified by flash chromatography on silica (40% ethyl acetate in hexane) to yield a resin: MS (m+1)=459.2; H NMR (500 MHz, CDCl3) 0.95 (m, 6H), 2.00 (m, 1H), 2.05 (m, 1H), 2.25 (m, 2H), 2.30 (m, 3H), 2.55 (t, 1H), 2.65 (t, 1H), 2.90 (m, 1H), 3.05 (d, 1H), 3.25 (d, 1H), 3.60 (m, 2H), 4.15 (m, 2H), 4.35 ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| body weight | aaaaa | aaaaa |
| body weight | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com