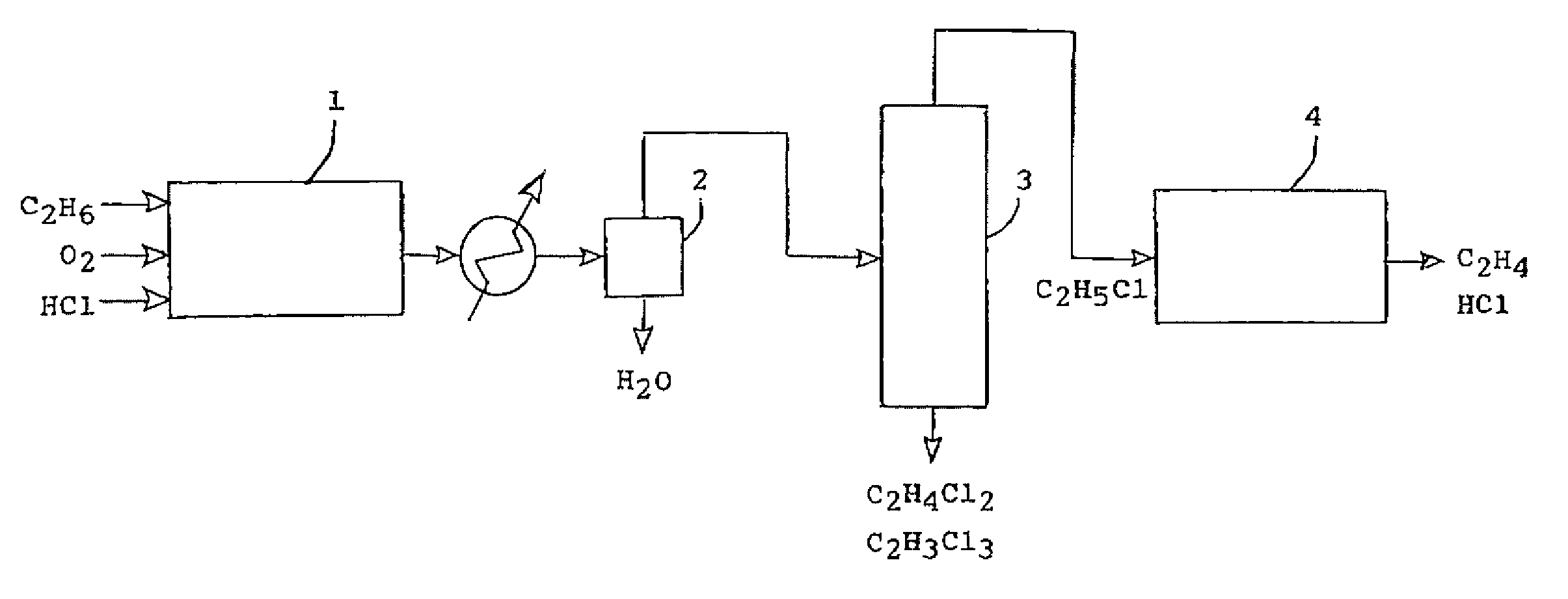
Process for producing ethylene by chlorination of ethane and dehydrochlorination of ethyl chloride
a technology of ethyl chloride and ethylene, which is applied in the preparation of chloride, hydrocarbon preparation catalysts, halogenated hydrocarbon preparations, etc., can solve the problems of ethylene production, and achieve the effect of robust features and favorable economics
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example
[0029]The catalyst composition for the oxychlorination of ethane to ethyl chloride was 40 mol percent copper chloride, 30 mol percent potassium chloride, 10 mol percent sodium chloride, and 20 mol percent lead chloride. The reaction was run at 370° C. At steady state conditions, 81.4 percent of the ethane was unreacted, 14.0 percent converted to ethyl chloride, 0.6 percent converted to 1, 1 dichloroethane, 2.2 percent to ethylene dichloride, 1.4 percent to trichloroethane and 0.4 percent to tetrachloroethane. No carbon monoxide or carbon dioxide was detected. The ethyl chloride was cracked over a silica alumina catalyst at 350° C. to give a near quantitative yield of ethylene. The catalyst was 12.4 weight percent Al2O3 and 87.3 percent SiO2 and had a surface area of 300 m2 / g.
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperatures | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com