Antimicrobial peptides
a technology of antimicrobial peptides and peptides, which is applied in the field of new drugs, can solve the problems of toxicity or ability to lyse eukaryotic cells of antimicrobial peptides as antibiotics, and achieve the effect of reducing the likelihood of contracting a microbial infection and inhibiting microbial growth
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
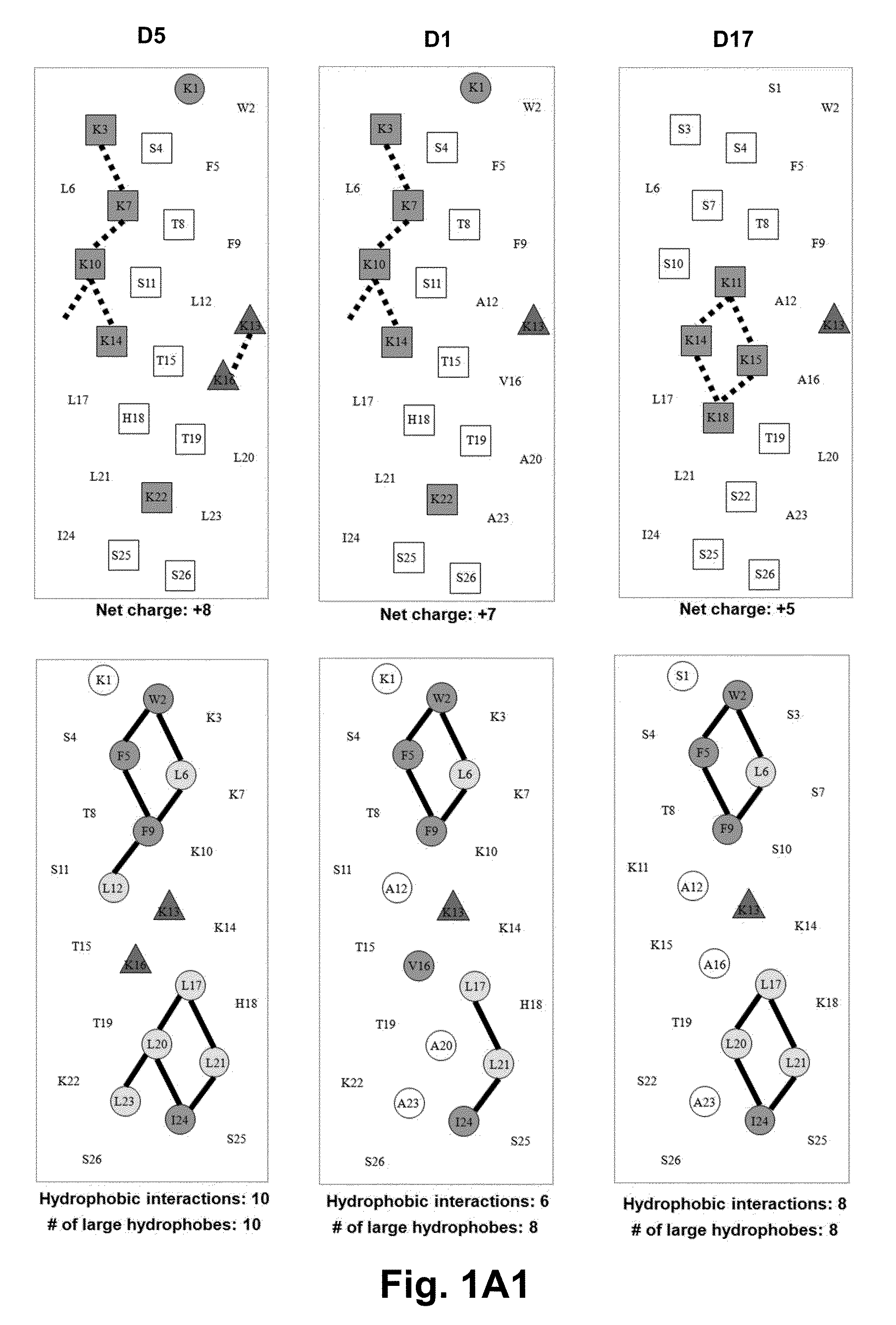
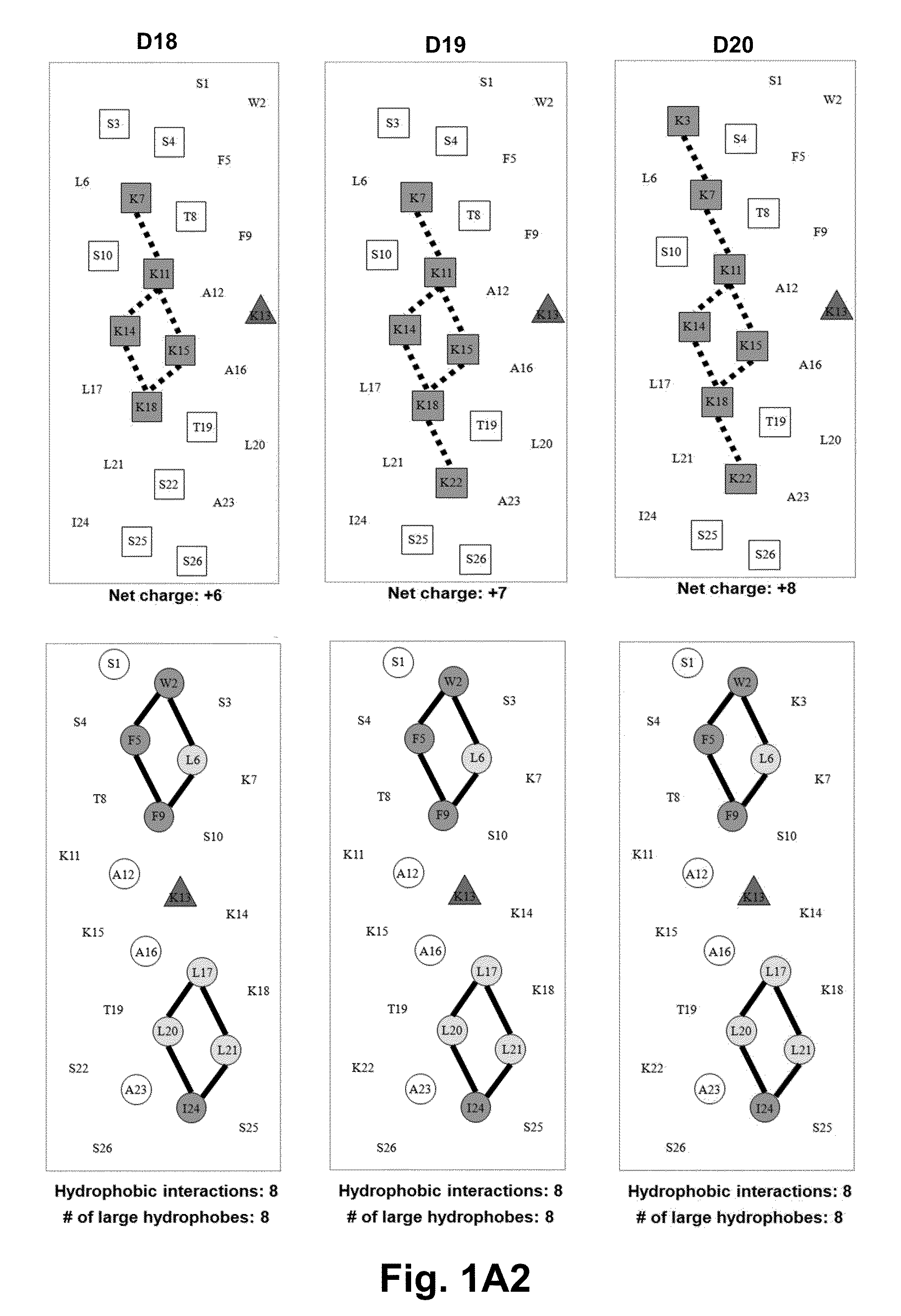
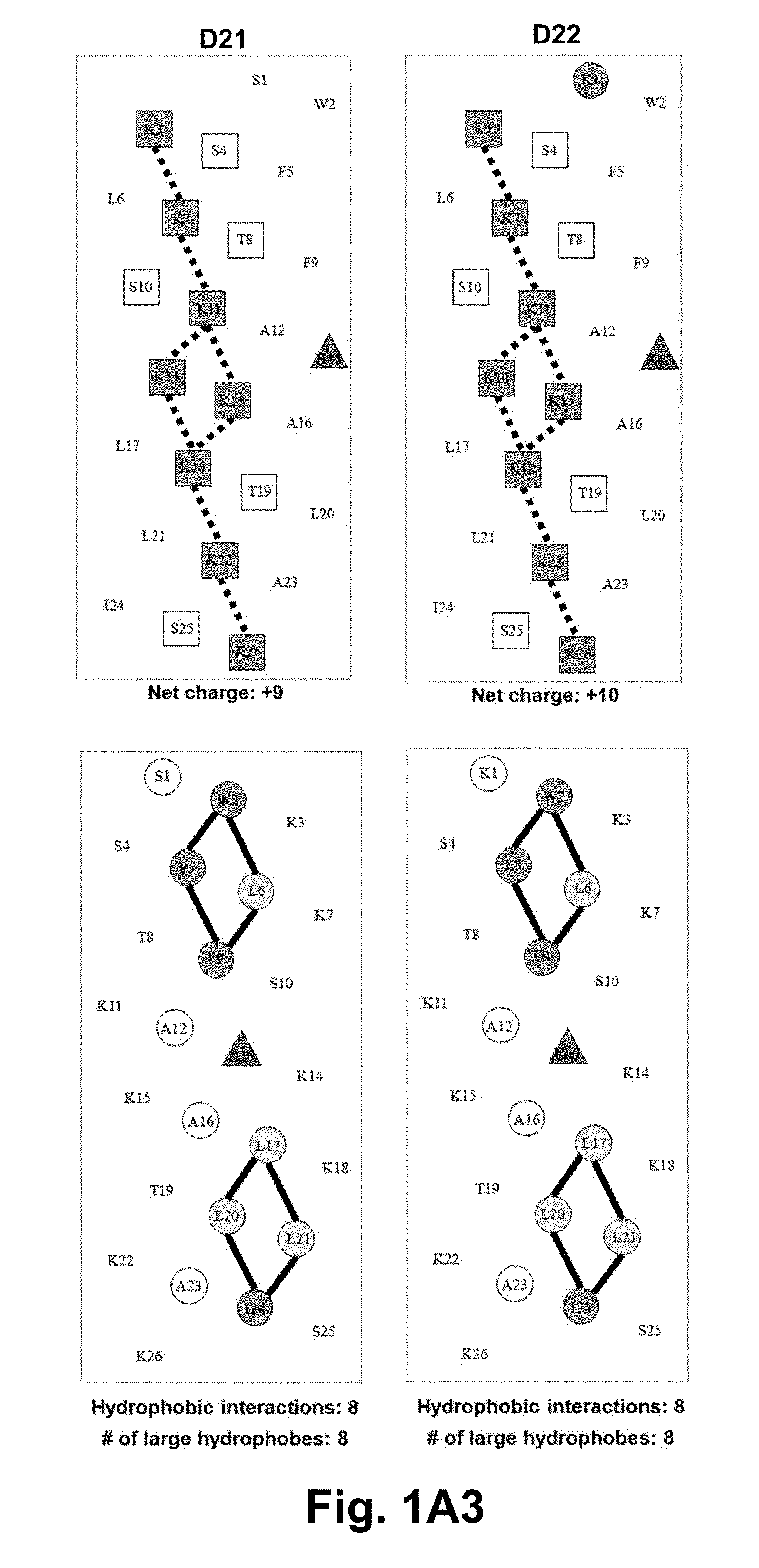
[0063]Due to the growing increase in antibiotic resistance, antimicrobial peptides (AMPs) have become important candidates as potential therapeutic agents. They have two unique features: a net positive charge of +2 or greater, or +5 to +11, owing to an excess of basic amino acids (Lys, Arg) over acidic amino acids (Asp, Glu); and an amphipathic nature, with a non-polar face and a polar face. The main target of such antimicrobial peptides is the cell membrane of microorganisms. A prior 26 amino acid residue peptide, V13K, showed that a single valine to lysine substitution (compared to its parent peptide) in the center of the non-polar face dramatically reduced toxicity and increased the therapeutic index. We then systematically substituted positively charged residues on the polar face to give a net positive charge from +5 to +11 as well as changing the relative location of these charged residues while maintaining the identical non-polar face for all analogs. We evaluated these peptid...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com