High throughput solid phase chemical synthesis utilizing thin cylindrical reaction vessels useable for biological assay
a technology of solid phase chemical synthesis and reaction vessel, which is applied in the field of combinatorial chemistry, can solve the problems of limited yield, limited quantity of synthesized compounds, and limited chemistries, and achieves convenient and reliable delivery of chemical samples, facilitate detailed study of chemical structure, and facilitate the effect of chemical structur
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
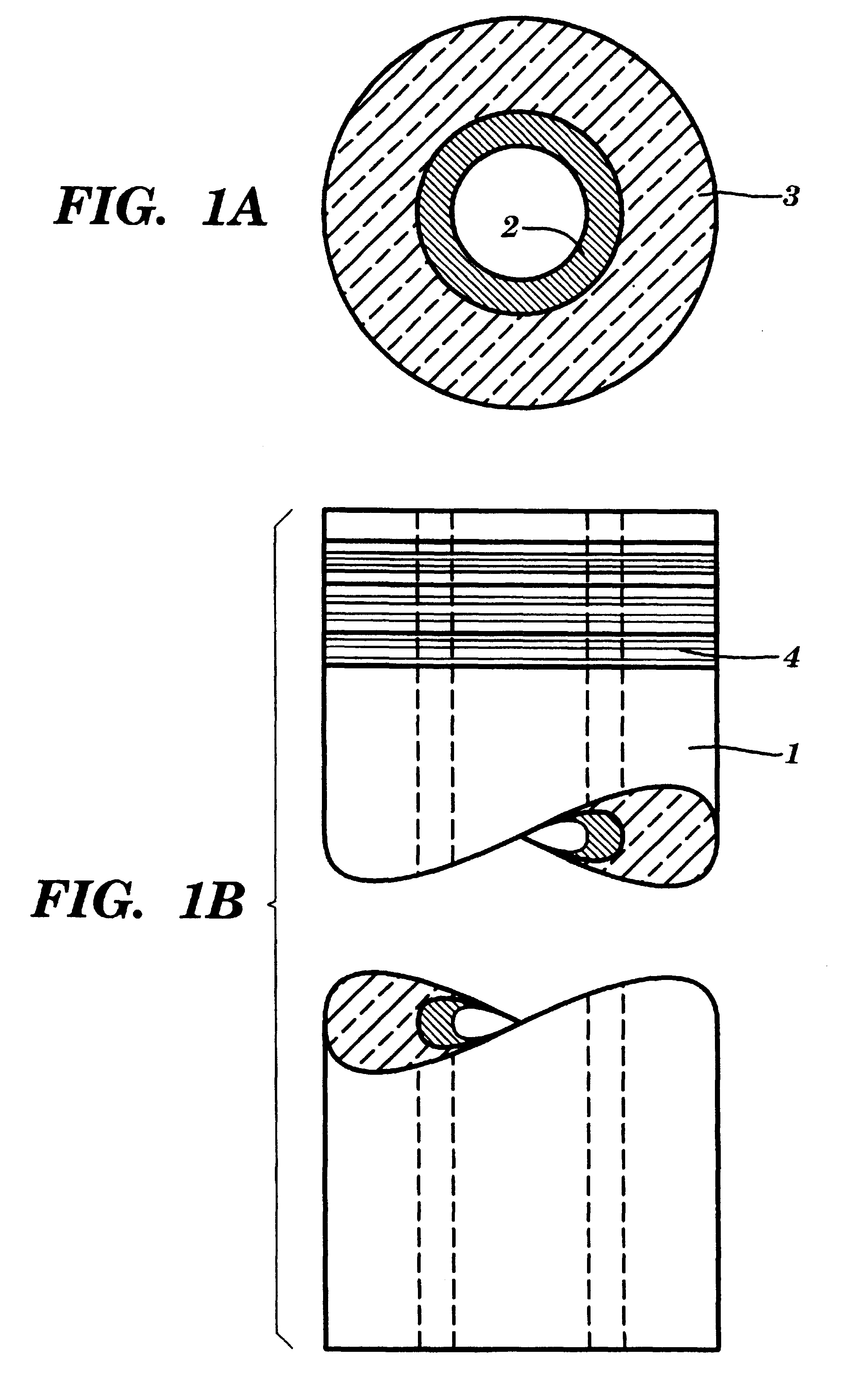
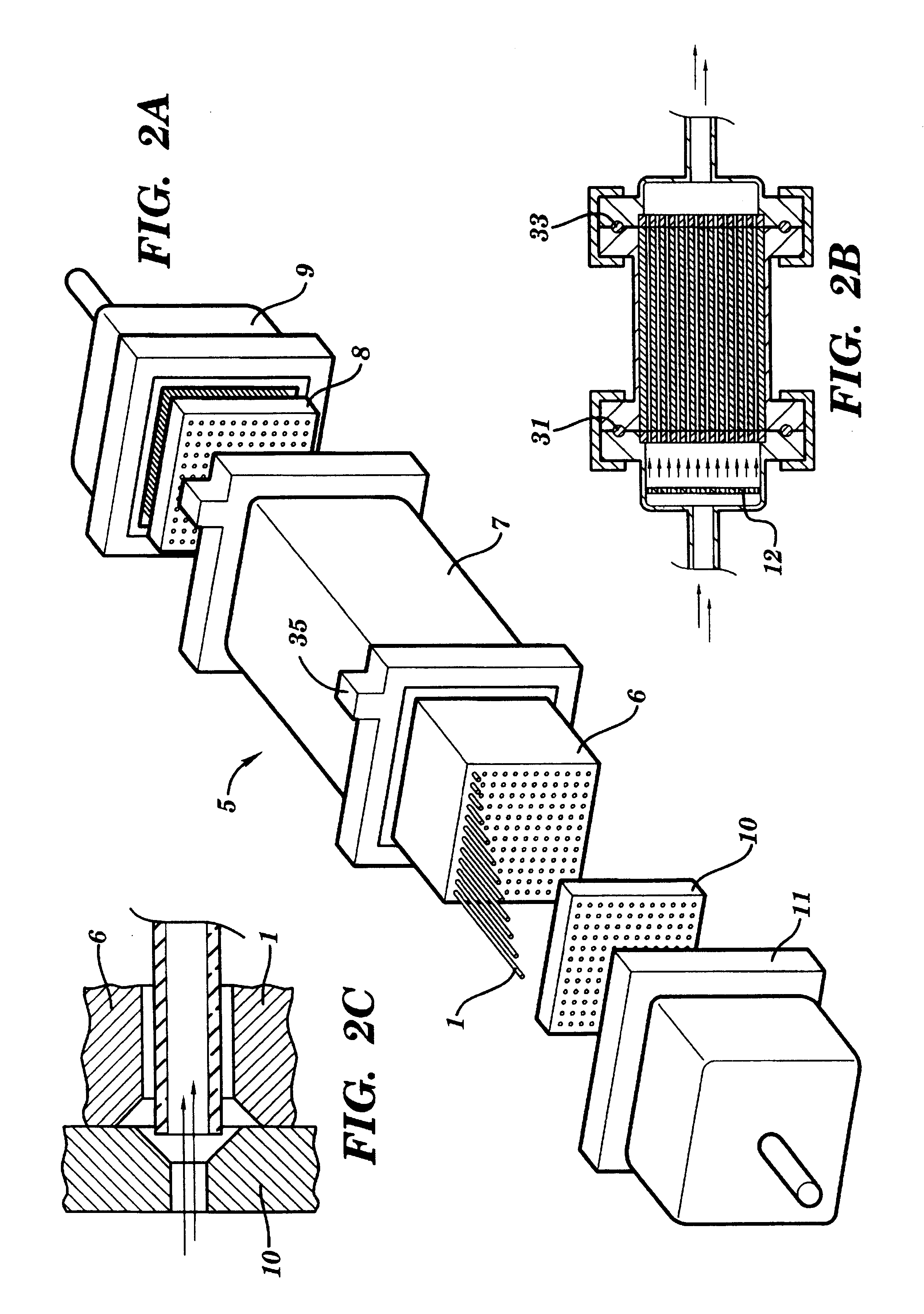
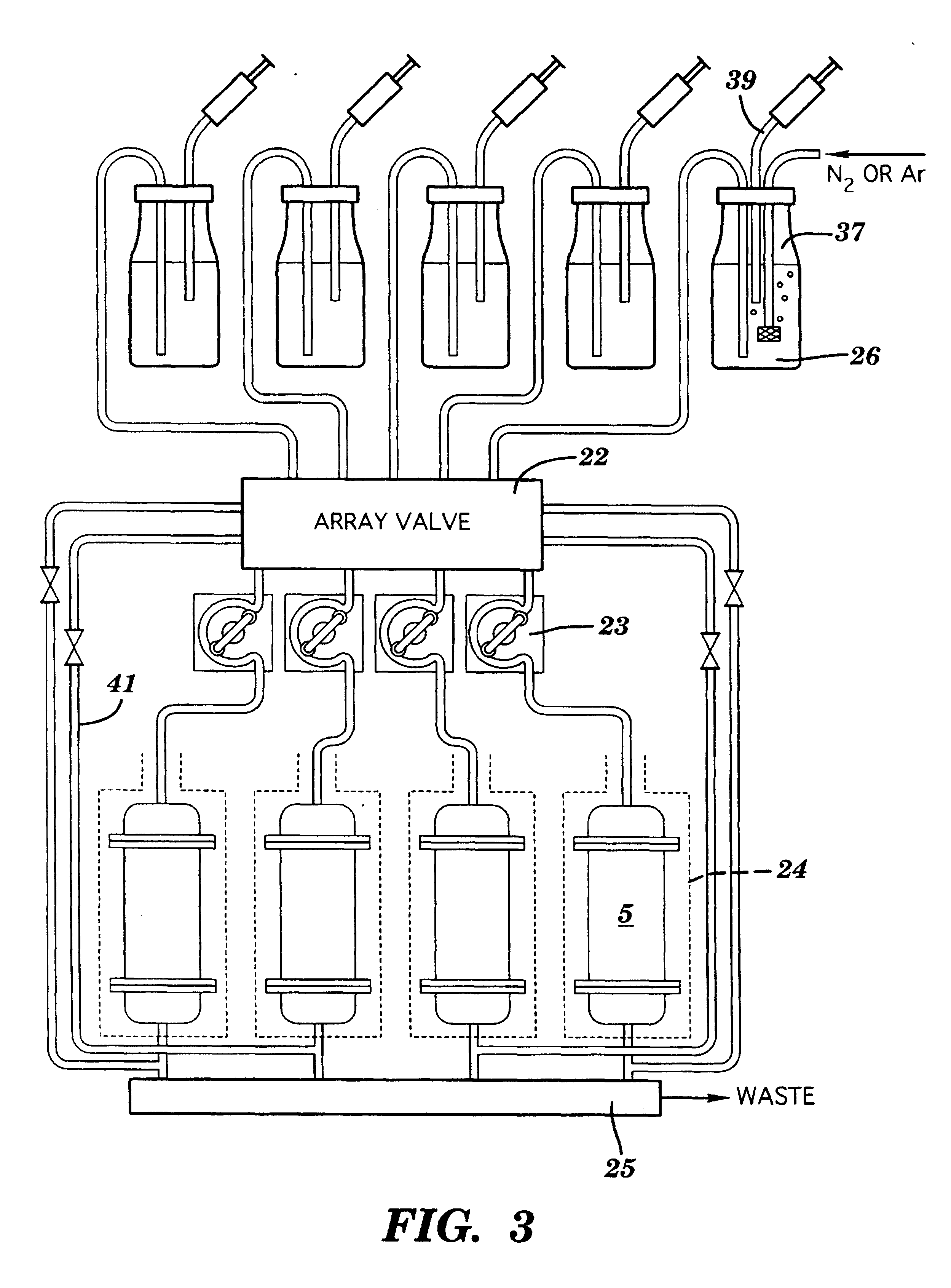
Referring to FIGS. 1A and 1B, a preferred reaction vessel 1 is shown. The reaction vessel 1, which is preferably disposable, is one component of a system for performing solid phase chemical synthesis in accordance with the principles of the present invention. Each vessel contains an inner reactive coating 2 (or inner reactive packing--see discussion of FIG. 7 below) to which molecules of a synthesized compound are attached, throughout the volume thereof, during such synthesis. Typically, the coatings or packings are chemical polymers such as cellulose, pore-glass, silica gels, polystyrene optionally cross-linked with divinylbenzene and optionally grafted with polyethylene glycol and optionally functionalized with amino, hydroxy, carboxy, or halo groups, grafted co-poly beads, polyacrylamide beads, latex, dimethylacrylamide optionally cross-linked with N,N'-bis-acryloyl ethylene diamine, glass coated with hydrophobic polymers, etc. Preferably, the coating or packing is divinylbenzene...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thick | aaaaa | aaaaa |
| temperatures | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com