Optochemical sensor and method for production
a technology of optochemical sensors and production methods, applied in the field of optochemical sensors, can solve the problems of hardly being able to detect small changes in the thickness of thin layers, and achieve the effect of reducing the difficulty of detection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
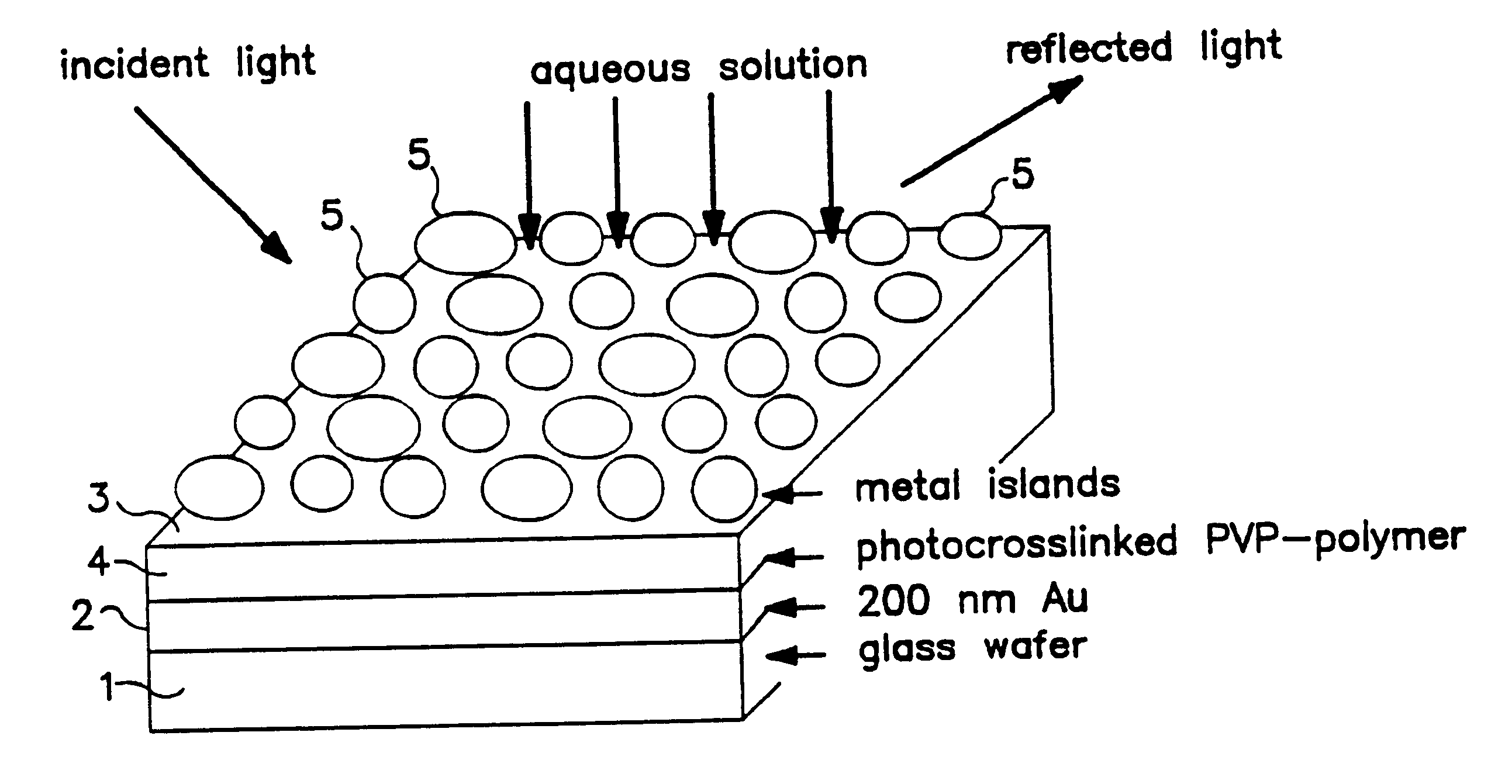
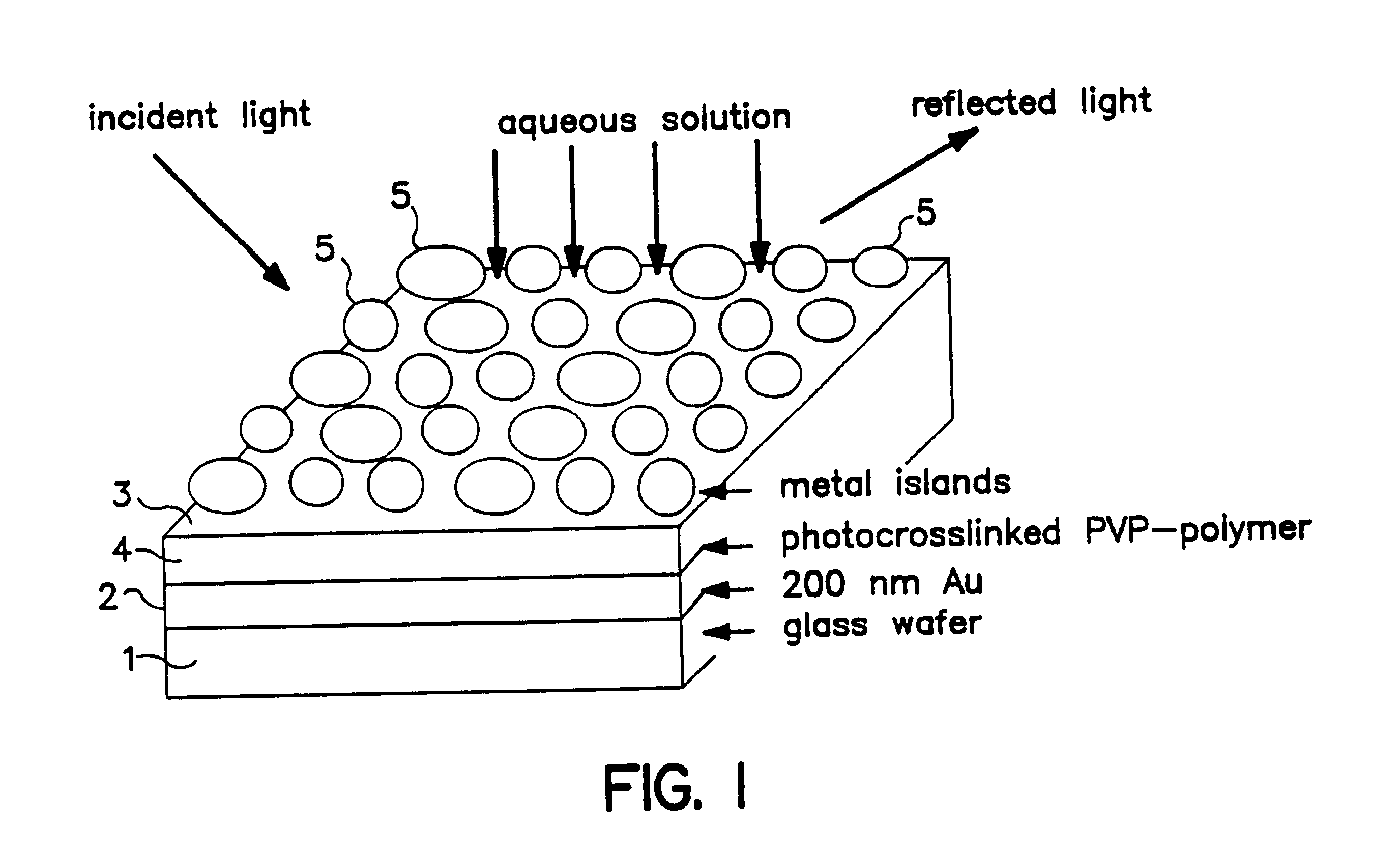
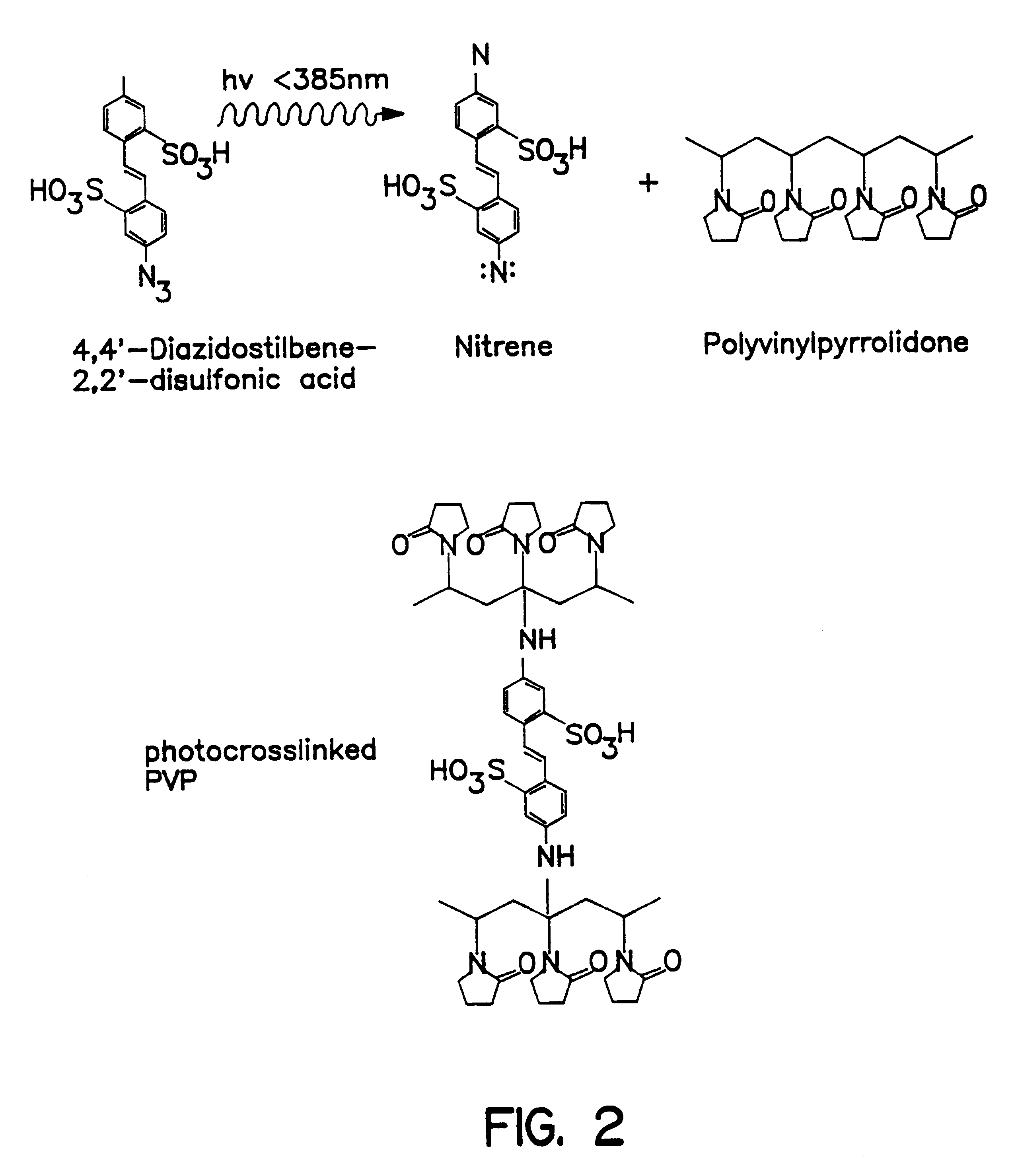
The optochemical sensor of FIG. 1 embodiment comprises a substrate layer 1, e.g., made of a glass wafer and a 200 nm gold coating as a mirror layer 2. Between the mirror layer 2 and a film 3 consisting of a plurality of islands 5 of electrically conductive material a reactive matrix layer 4 is situated. The matrix layer 4 is made of a material that is capable of swelling. The reactive matrix layer 4 can be derived e.g., via crosslinking reaction of polyvinylpyrrolidone with 4,4'-dazidostilbene-2,2-disulfonic acid as shown in FIG. 2.
FIG. 3 shows the influence of pH on polymer swelling and shrinking using Na.sub.2 HPO.sub.4 / NaH.sub.2 PO.sub.4 buffer (200 mM) over the pH 4.3 to 9.3 range.
FIG. 4 shows the absorbance of such a sensor at a wavelength of 720 nm due to pH induced swelling and shrinking of the reactive matrix layer over the pH 4.3 to 7.0 range.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com