Microparticle carriers of maximal uptake capacity by both M cells and non-M cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
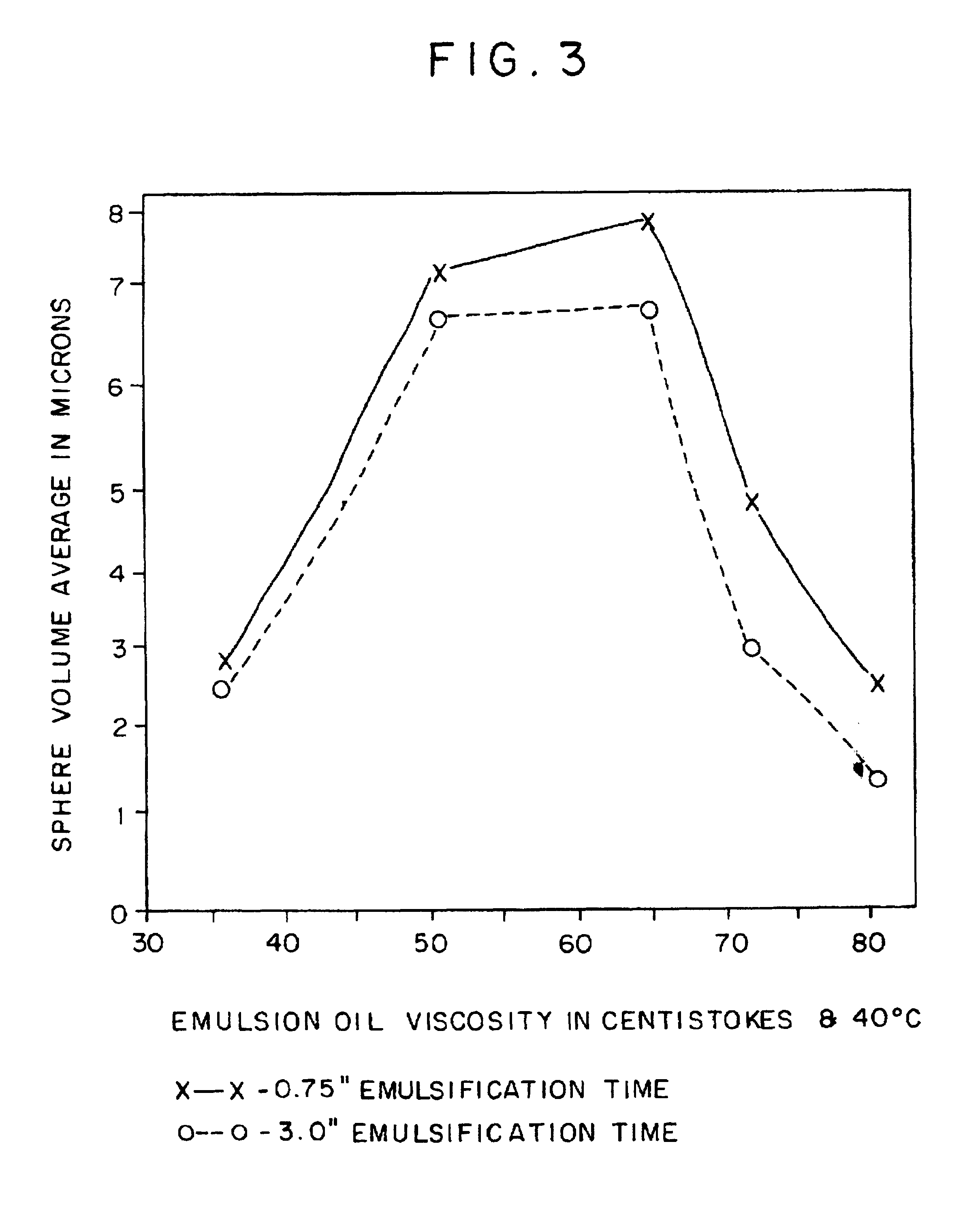
Image
Examples
example
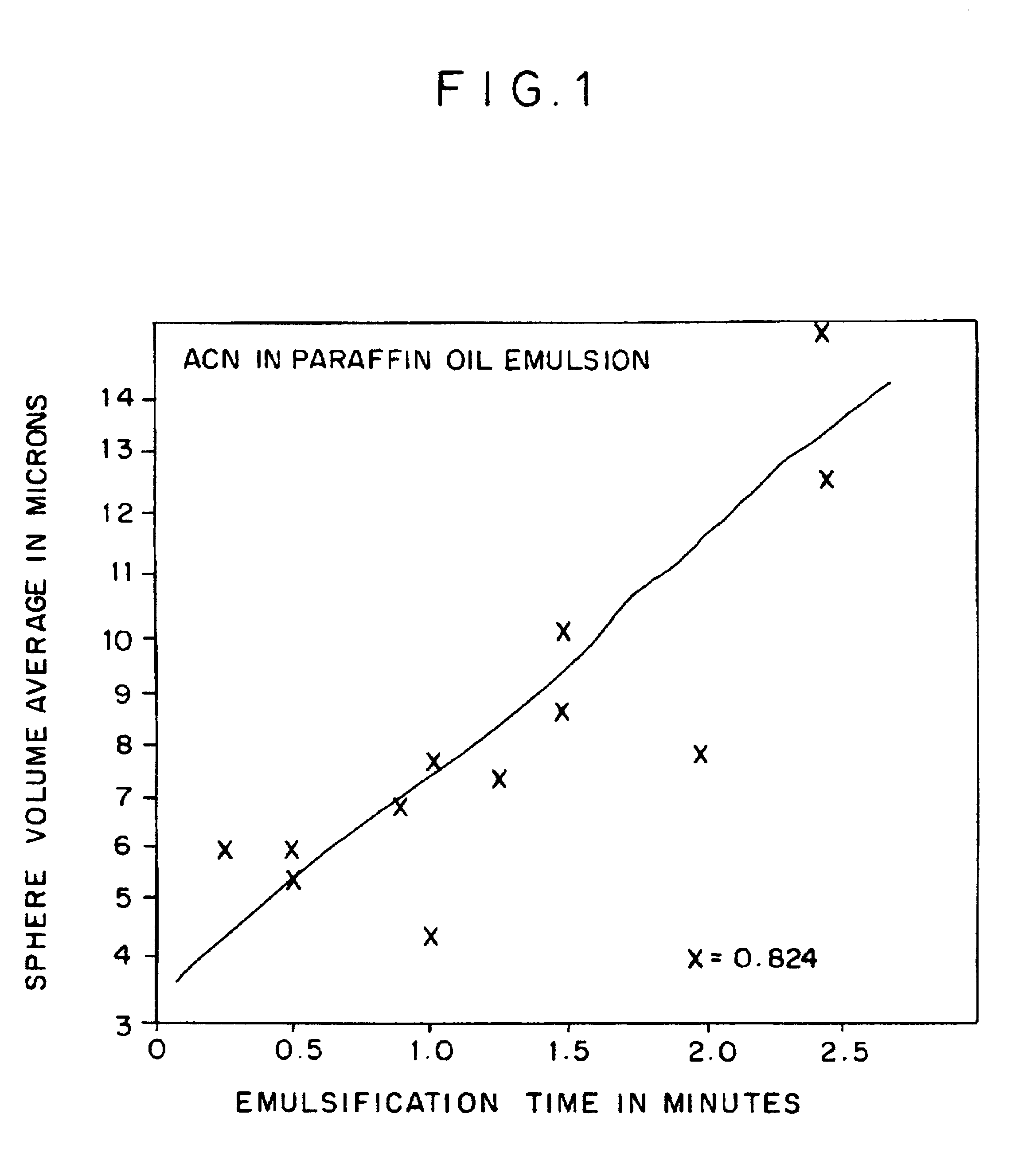
Solvent Extraction
[0039]Preparation of Freeze-Dried Antigen-Sucrose Matrix
[0040]Materials
[0041]8 ml water
[0042]80 mg sucrose
[0043]20 mg purified antigen / active
[0044]The freeze-drier is turned on and the temperature is set at −25 degrees.
Preparation of the Antigen-Sucrose Matrix
[0045]The antigen / active is placed in a 20 ml capacity plastic vial to which water and sucrose are added.
[0046]The dispersion is then flash freezed by gently swirling the vial (without the cap) in liquid nitrogen for about one half of an hour.
[0047]After about 1000 minutes the temperature is elevated to about +5 degrees for 500 minutes (8.33 hours) and then elevated to about +20 degrees for 1000 minutes (16.67 hours), and the vial is removed.
Preparation of Polymerized Lactide Glycolide (PLG) Solution
[0048]The PLG is removed from the freezer and allowed to come to room temperature.
[0049]About 2.8 g of acetonitrile is weighed into a 20 ml capacity glass vial and set aside.
[0050]After the polymer reaches room tem...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Length | aaaaa | aaaaa |
| Particle size distribution | aaaaa | aaaaa |
| Particle size distribution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com