Design of molecules
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
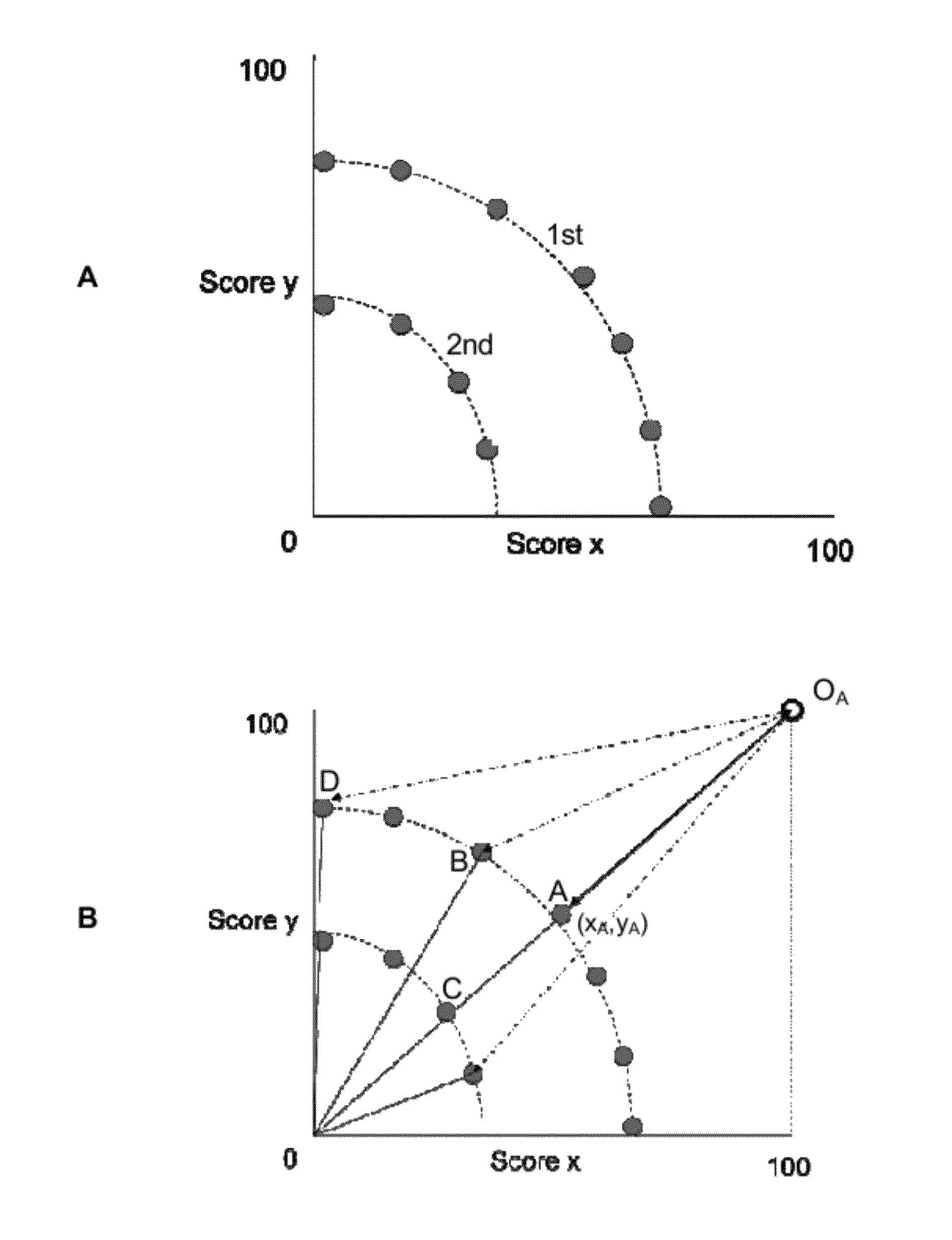
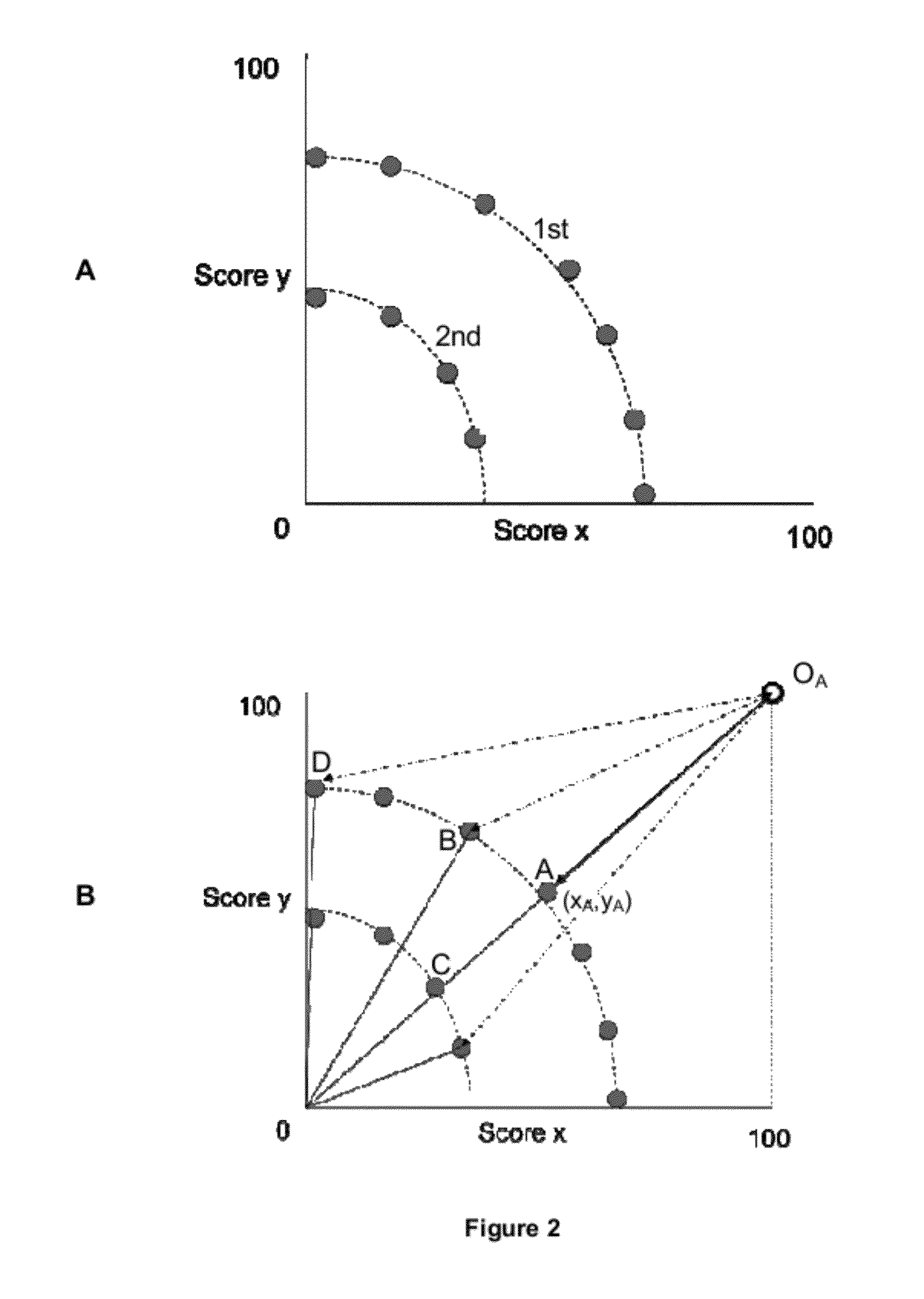
Image
Examples
example 1
Optimisation of Tadalafil from Lead Compound
[0181]This example demonstrates the optimisation and selection of a new drug from a lead compound: specifically, the design, selection and optimisation of the approved drug tadalafil from its lead compound.
[0182]Tadalafil, chemical name (6R,12aR)-6-(1,3-Benzodioxol-5-yl)-2-methyl-2,3,6,7,12,12a-hexa hydropyrazino[1′,2′:1,6]pyrido[3,4-b]indole-1,4-dione, is a phosphodiesterase 5 (PDE-5) inhibitor, which is approved for the treatment of erectile dysfunction. The optimisation of tadalafil from its hydantoin lead compound, 2-Butyl-5-(4-methoxyphenyl)-5,6,11,11a-tetrahydro-1H-imidazo[1′,5′: 1,6]pyrido[3,4-b]indole-1,3(2H)-dione, is described Daugan A. et al. (2003), J. Med. Chem., 46(21), pp 4533-4542 (Compound 2a). Daugan A. et al. report that a medicinal chemistry strategy identified the optimum compound, tadalafil through the synthesis of at least 48 compounds in a series of structure activity exercises from the lead compound.
[0183]To demons...
example 2
Selective Optimisation of a Side Activity of a Known Drug into the Primary activity of a New Compound
[0224]The most successful strategy to date for drug discovery has been the modification of one drug to create a new drug. The molecular exploration of existing compounds by structural modification to create new compounds with new activities has been proposed as a strategy, for example, by Wermurth (2004, J. Med. Chem., 47(6), 1303-1314; and Wermuth, (2006), Drug Discovery Today, 11(3 / 4), 160-164). This strategy, i.e. where the secondary weak activity of a drug for a target is optimised to create a new compound with improved activity against that side target, has been termed “Selective Optimisation of Side Activities” (SOSA). Typically the original primary activity is reduced as a result of the optimisation process.
[0225]In this Example compounds with new biological activity profiles are designed from an existing known drug, donepezil, an acetylcholinesterase inhibitor approved for th...
example 3
Demonstration of the Algorithm to Design Against Anti-Targets to Improve Selectivity
[0269]In many drug discovery programmes it is often necessary to improve the ratio of the affinity of a lead compound for a desired target molecule over an undesired target molecule. This ratio of the affinity for an undesired target over a desired target is referred to as the selectivity of a compound. Since achieving selectivity can be such an important problem to be solved, in this Example we applied the algorithm of the invention to the design of compounds that have increased selectivity for desired targets over undesired targets.
[0270]The seven isoindole analogues that were selected in Example 2 (i.e. GFR-VII-274, GFR-VII-281, GFR-VII-285, GFR-VII-287, GFR-VII-280 GFR-VII-290, and GFR-VII-273) to have improved binding activity for dopamine D2 demonstrated a significantly increased affinity for the dopamine D2 receptor over the starting compound donepezil. However, activity against other receptor...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Volume | aaaaa | aaaaa |
| Mass | aaaaa | aaaaa |
| Mass | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com