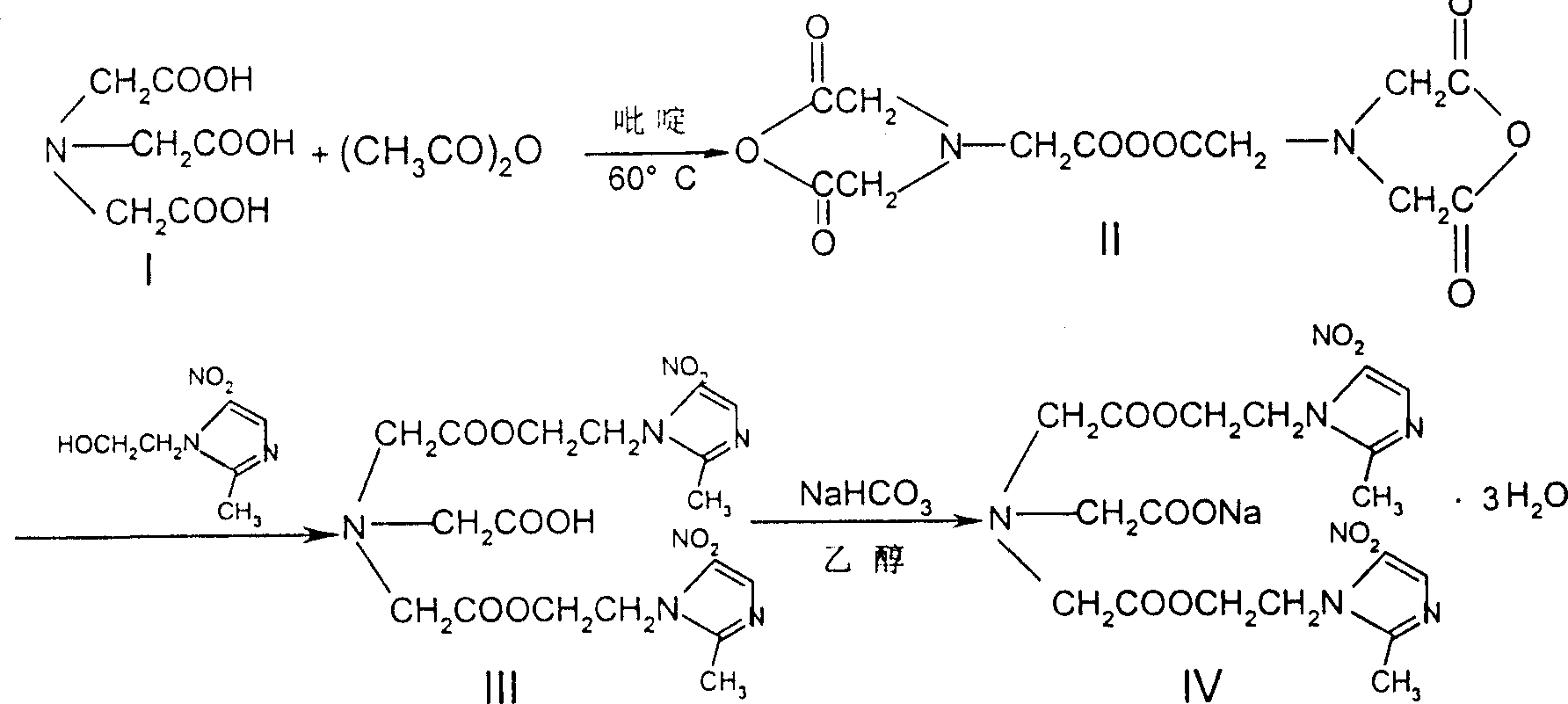
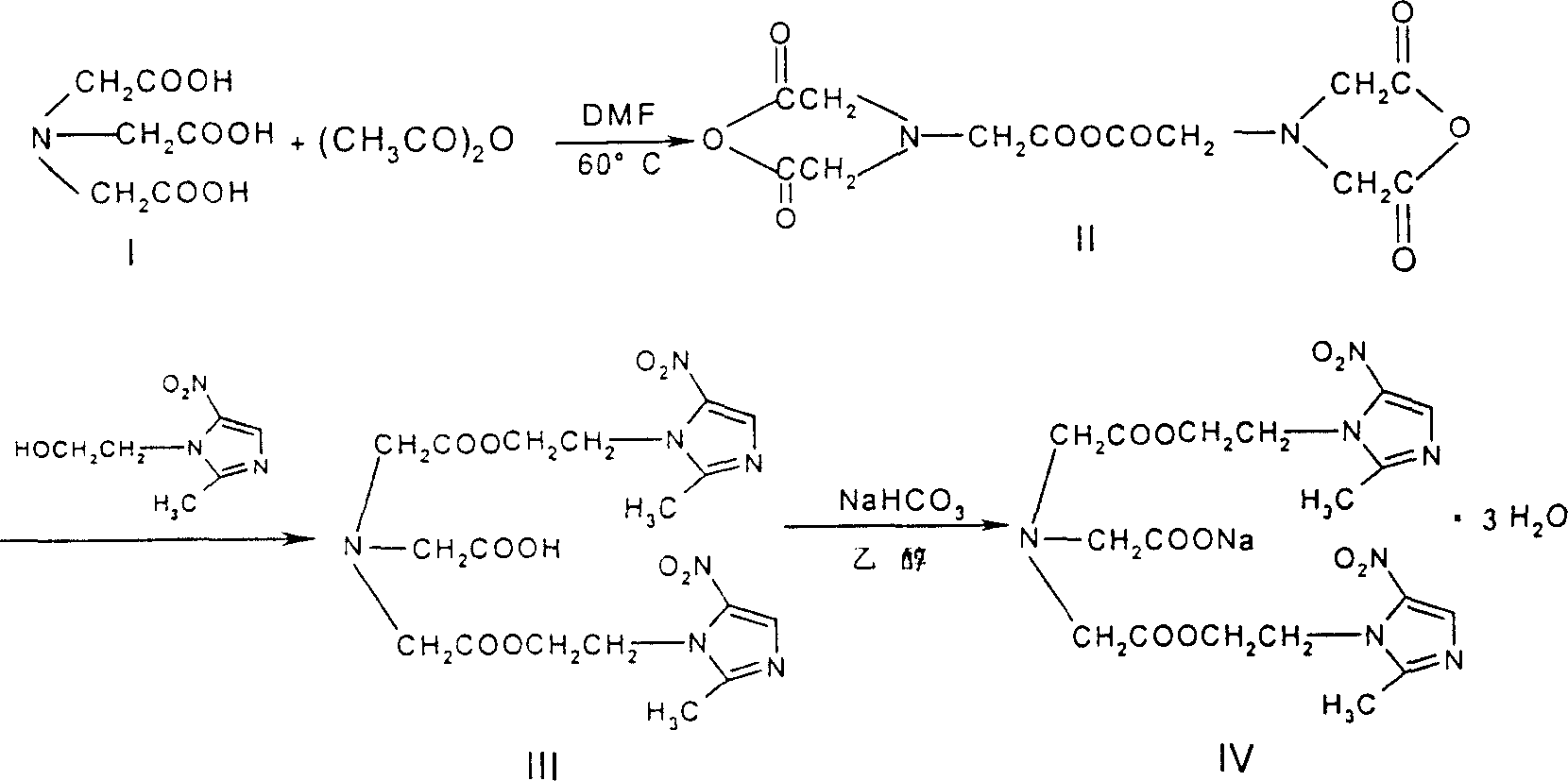
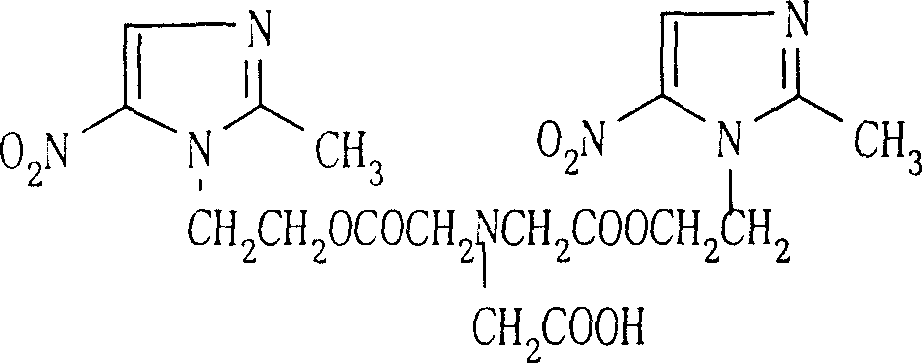Process for synthesis of sodium glycididazole
A technology of sodium glycidazole and glycidazole is applied in the field of medicinal chemistry, and can solve the problems of mother liquor treatment, difficulty in recovery, unpleasant smell, and high pyridine unit consumption.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0145] Embodiment 1, the preparation-esterification reaction of glycidiazole
[0146] A, in 100 liters of reactors, add 6.0 kilograms of nitrilotriacetic acid (1, 31.4mol), 30 kilograms of DMF and 12 kilograms of acetic anhydride (118mol), in 50 ℃ of insulated stirring reactions 6 hours;
[0147] B, add 14.0 kilograms (81.8mol) of metronidazole in the reactor, continue to insulate and stir the reaction for 7 hours at 40°C;
[0148] C. After the reaction is over, the interlayer passes cold water to cool the reaction feed liquid;
[0149] D. Cool the reaction liquid to 30°C, add purified water, stir until a precipitate precipitates out, and cool it down (<5°C) to precipitate crystals;
[0150] E. The precipitate is dried and dehydrated, washed with water until neutral;
[0151] F. After centrifugation, the crude product of glycididazole was obtained, with a wet weight of 9.18 kg and a yield of 47.0%.
Embodiment 2
[0152] Embodiment 2, the preparation-esterification reaction of glycidiazole
[0153] A, add 7.0 kilograms of nitrilotriacetic acid (1, 36.6mol), 30 kilograms of DMF and 16 kilograms of acetic anhydride (157mol) in 100 liters of reactors, in 65 ℃ of insulated stirring reactions 4 hours;
[0154] B, add 18.0 kilograms (105mol) of metronidazole in the reaction kettle, continue insulated stirring reaction at 60 ℃ for 5 hours;
[0155] C. After the reaction is over, the interlayer passes cold water to cool the reaction feed liquid;
[0156] D. Cool the reaction liquid to 30°C, add purified water, stir until a precipitate precipitates out, and cool it down (<5°C) to precipitate crystals;
[0157] E. The precipitate is dried and dehydrated, washed with water until neutral;
[0158] F. After centrifugation, the crude product of glycididazole was obtained, with a wet weight of 11.3 kg and a yield of 49.6%.
Embodiment 3
[0159] Embodiment 3, the preparation-esterification reaction of glycidiazole
[0160] A, add 8.0 kilograms of nitrilotriacetic acid (1, 41.8mol), 30 kilograms of DMF and 20 kilograms of acetic anhydride (196mol) in 100 liters of reactors, in 80 ℃ of insulated stirring reactions 2 hours;
[0161] B, add 22.0 kilograms (128mol) of metronidazole in the reactor, continue to insulate and stir the reaction for 3 hours at 80°C;
[0162] C. After the reaction is over, the interlayer passes cold water to cool the reaction feed liquid;
[0163] D. Cool the reaction liquid to 30°C, add purified water, stir until a precipitate precipitates out, and cool it down (<5°C) to precipitate crystals;
[0164] E. The precipitate is dried and dehydrated, washed with water until neutral;
[0165] F. After centrifugation, the crude product of glycididazole was obtained, with a wet weight of 13.4 kg and a yield of 51.4%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com