Interfusion protein of antineoplastic genetic engineering composed of target short peptide of receptor of epidermal growth factor and Lidamycin
A fusion protein, lidamycin technology, applied in the direction of antitumor drugs, peptide/protein components, medical preparations containing active ingredients, etc., to achieve the effects of strong penetration, good stability and strong killing effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
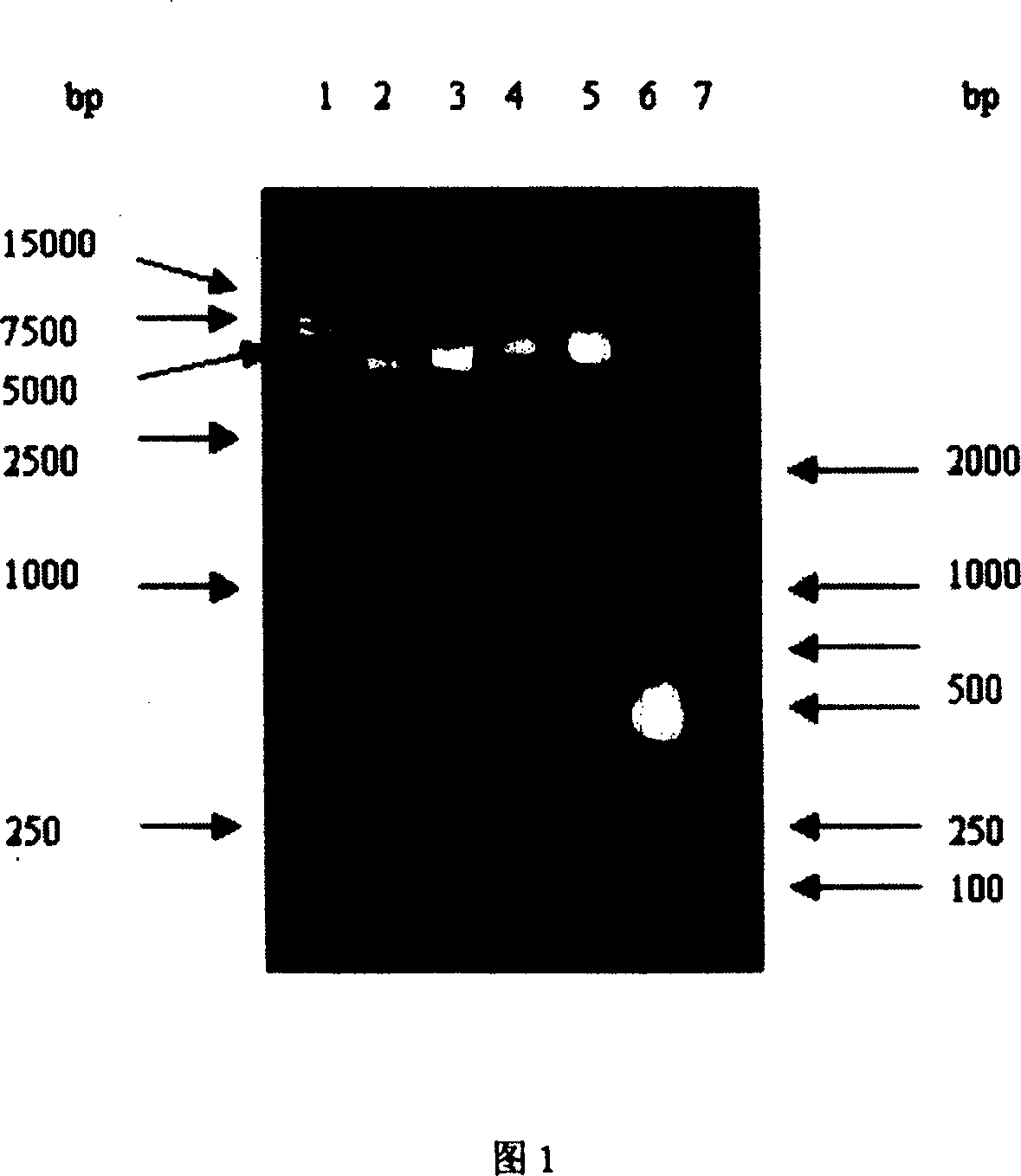
Image
Examples
Embodiment 1
[0091] Example 1. Construction of the expression vector pET30SGLDP containing the short peptide SG / lidamycin prosthetic protein LDP gene for EGFR targeting binding
[0092] L1 upstream 5'GA CAT ATG AAC CCC GTG GTG GGC TAC ATC GGT GAA CGT CCT CAG
[0093] Nde I
[0094] TAT CGT GAC CTG GGT GGA GGC GGT TCA GCG CCC GCC TTC TCC GTC3’
[0095] 5'GTTA downstream of R1 CTC GAG GCC GAA GGT CAG AGC CAC GTG3'
[0096] Xho I
[0097] L2 upstream 5'GAATCCA CAT ATG AAA TAC CTG CTG CCG ACC GCT GCT GCT GGT CTG
[0098] Nde I
[0099] CTG CTC CTC GCT GCC CAG CCG GCG ATG GCC ATG GCC AAC CCC GTG
[0100] GTG GGC TAC3'
[0101] 5'GTTA downstream of R2 CTC GAG GCC GAA GGT CAG AGC CAC GTG3'
[0102] Xho I
[0103] Using the plasmid pIJ1027GRGDS (containing the LDP gene, constructed by our laboratory, which can be provided to the public and issue relevant certificates) as a template, the prime...
Embodiment 2
[0105] Embodiment 2. Fusion protein SG-LDP is in Escherichia coli BL21 (DE3) star TM Induced expression in
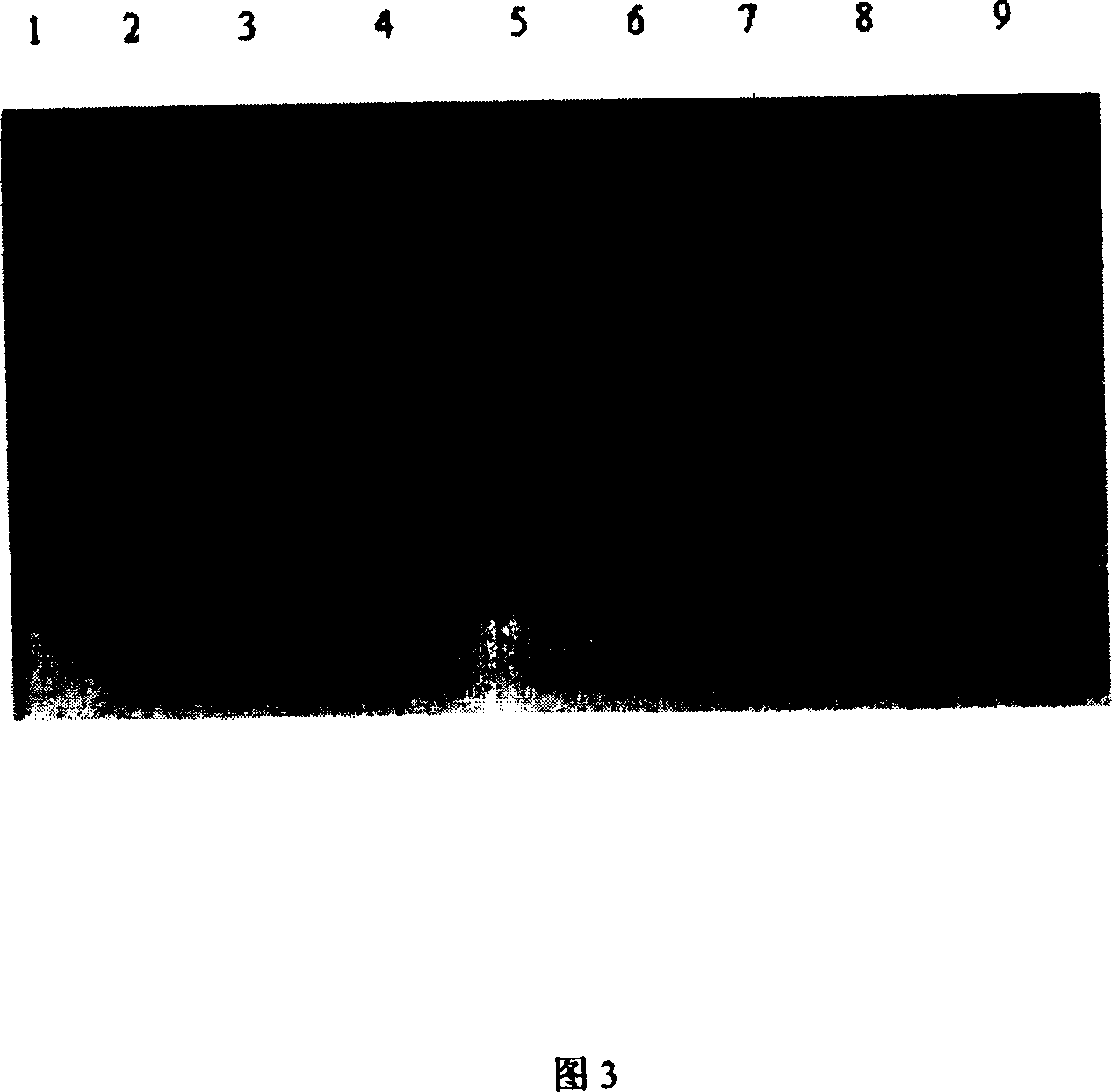
[0106] The expression vector used in the present invention is Escherichia coli expression system E.coli BL21(DE3)star TM , for Invitrogen products. Transform Escherichia coli BL21(DE3)star with the constructed recombinant plasmid TM To obtain recombinant transformed bacteria, pick a single clone colony from the LB plate and inoculate it into the LB medium containing 50 μg / ml kanamycin, and cultivate overnight at 37° C. with shaking at 180 rpm. On the next day, replant with 2-3% inoculum, shake culture at 37°C for 2-3 hours to OD 600 About 0.8-1.0, add isopropyl-β-D-thiogalactopyranoside (IPTG) at a final concentration of 0.05mmol / L to the culture, and induce for 8 hours. The 15% SDS-PAGE analysis results (Fig. 3) show that there is an obvious exogenous protein expression band at about 14kDa in the induced Escherichia coli in the culture medium, and the expression am...
Embodiment 3
[0107] Example 3. Affinity chromatography purification of fusion protein SG-LDP and its separation and preparation
[0108] The supernatant of the bacterial cell fermentation broth induced to express was collected, and ammonium sulfate was added at a concentration of 113 g / L at 4° C. under the condition of slow stirring. Stand still at 4°C for 1 hour, centrifuge at 10,000 g for 20 minutes at 4°C, and collect the supernatant. Ammonium sulfate was added to the supernatant at 4°C under slow stirring to make the final concentration 390 g / L. Stand still at 4°C for half an hour, centrifuge at 10,000g for 20 minutes at 4°C, and collect the precipitate. Use 2ml 1×Ni-NTA Binding Buffer (50mMNaH 2 PO 4 , 300mM NaCl, 10mM imidazole, pH8.0) were dissolved and dialyzed with the same solution.
[0109] Ni-NTA His-Bind resin (Novagen) was loaded into the chromatographic column, equilibrated with 1×Ni-NTA Binding Buffer, and the dialyzed sample was filtered through a 0.45um filter membran...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com