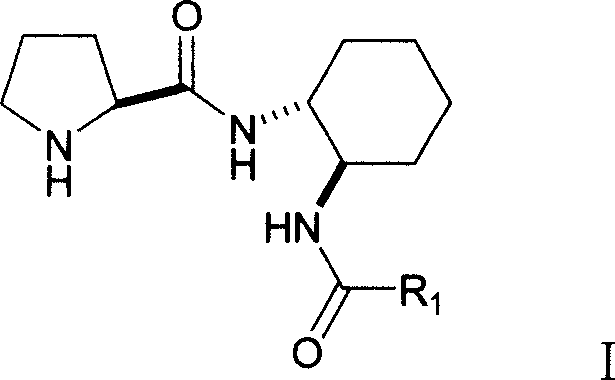
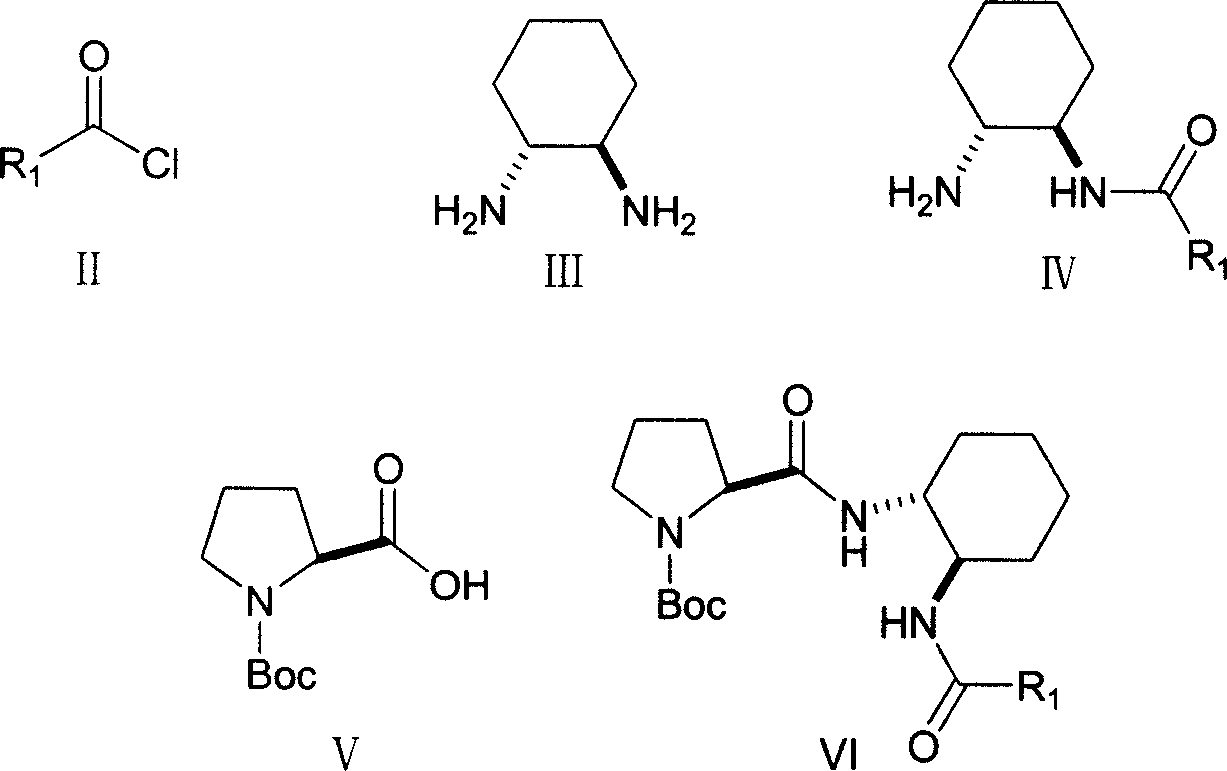
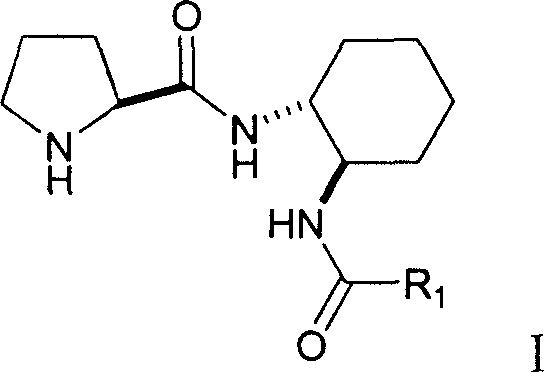
Double functional group L-proline derivative with adjustable catalytic activity and its prepn
A technology of proline and derivatives, which is applied in the field of preparation of bifunctional L-proline derivatives, can solve the problems of general selectivity and achieve good catalytic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Preparation
[0018] Under the protection of room temperature and dry nitrogen, 3.42 g (30 mmol) (1R, 2R)-diaminocyclohexane was dissolved in 20 mL of dichloromethane solution, and the mixture was cooled to 0° C. and stirred vigorously. Dissolve 10mmol of 4-methylbenzoyl chloride in 10mL of dichloromethane and add it dropwise to the reaction system: After the addition is completed, warm to room temperature and react for 12 hours; after the reaction, the solvent is evaporated, and the residue is added dropwise to the silica gel column , And perform column chromatography separation with ethyl acetate / methanol, the volume ratio of ethyl acetate to methanol is 3:1, and the chromatographic liquid is removed under reduced pressure to obtain the amide intermediate.
[0019] Dissolve 2.15g (10mmol) of Boc-protected L-proline and 2.07g (10mmol) of N,N'-dicyclohexylcarbimide in 20mL of dichloromethane and vigorously stir at 0°C for 30 minutes. 1.86g (8mmol) of amide intermediate in...
Embodiment 2
[0026] Preparation
[0027] Under the protection of room temperature and dry nitrogen, 3.42 g (30 mmol) (1R, 2R)-diaminocyclohexane III was dissolved in 20 mL of dichloromethane solution, and cooled to 0° C. and stirred vigorously. Dissolve 10mmol of 4-chlorobenzoyl chloride in 10mL of dichloromethane and add it dropwise to the reaction system; after the dropwise addition is completed, warm to room temperature and react for 12 hours; after the reaction, the solvent is evaporated, and the residue is carried out with ethyl acetate / methanol Column chromatography separation (the method is the same as in Example 1) to obtain the amide intermediate.
[0028] Dissolve 2.15g (10mmol) of Boc-protected L-proline and 2.07g (10mmol) of N,N'-dicyclohexylcarbimide in 20mL of dichloromethane and vigorously stir at 0°C for 30 minutes. 10 mL of a dichloromethane solution of 2.02 g (8 mmol) of the amide intermediate was added dropwise to the reaction system. After the dropwise addition, it was ra...
Embodiment 3
[0035] Preparation
[0036] Under the protection of room temperature and dry nitrogen, 3.42 g (30 mmol) (1R, 2R)-diaminocyclohexane was dissolved in 20 mL of dichloromethane solution, and cooled to 0° C. and stirred vigorously. 10mmol of trifluoroacetyl chloride was dissolved in 10mL of dichloromethane and added dropwise to the reaction system. After the addition, the temperature was raised to room temperature and reacted for 12 hours. After the reaction, the solvent was evaporated, and the residue was subjected to column chromatography with ethyl acetate / methanol (the method is the same as in Example 1) to obtain the amide intermediate.
[0037]Dissolve 2.15g (10mmol) Boc-protected L-proline and 2.07g (10mmol) N,N'-dicyclohexylcarbimide in 20mL dichloromethane and stir vigorously for 30 minutes at 0°C. 10 mL of a dichloromethane solution of 1.68 g (8 mmol) of the amide intermediate was added dropwise to the reaction system. After the addition, the temperature was raised to room...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com