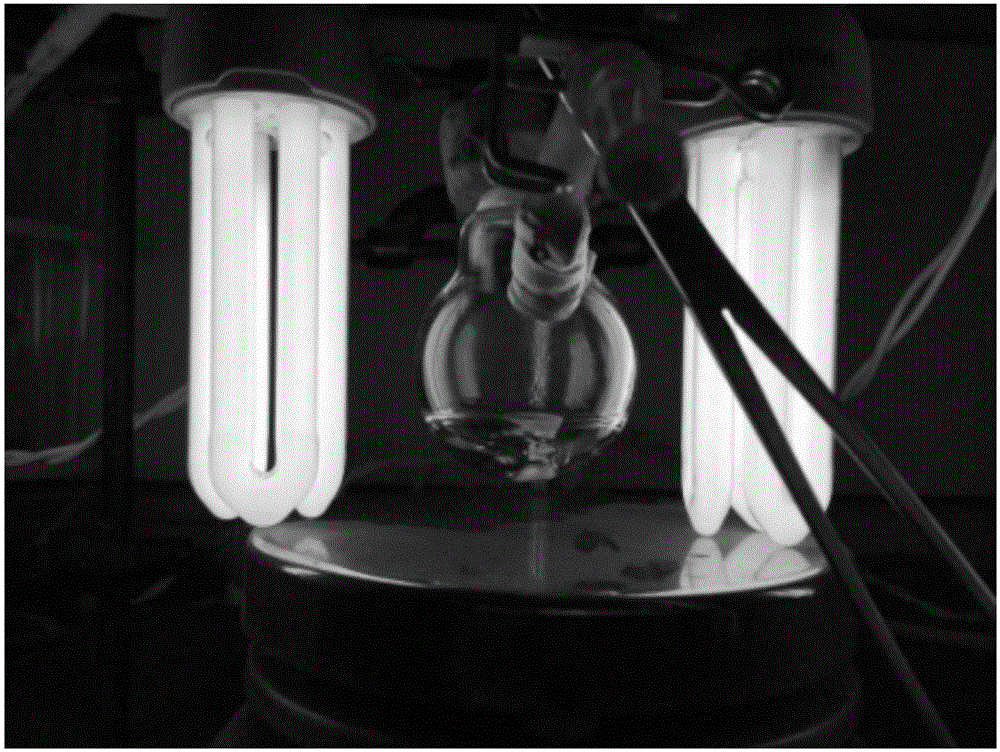Initiation system for photo-polymerization of active free radicals of methacrylate monomers
A technology of methacrylic acid and initiating system, applied in the field of initiating system, can solve problems such as few advantages and complex structure, and achieve the effect of simple and easy preparation method and strong practicability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Embodiment one, the photopolymerization of MMA monomer under p-methoxybenzaldehyde / 1-iodoperfluorohexane initiation system
[0022] The mass fraction of monomer MMA in N,N-dimethylformamide (DMF) solvent (6.0g) was 24wt%. According to the molar ratio [MMA] / [1-iodoperfluorohexane] / [p-methoxybenzaldehyde] / [DMA]=100 / 2 / 5 / 5, respectively add the above-mentioned raw materials into 25mL single branch round bottom in the flask. seal. Refrigerated and evacuated three times, and finally introduced argon, so that the polymerization reaction was carried out under the irradiation conditions of two 23W compact energy-saving lamps in an inert atmosphere. Distance response devices such as figure 1 shown. At different time intervals, a small amount of samples were taken from the reaction system with a syringe, and the samples were precipitated three times with a mixture of methanol and water with a volume ratio of 7:3, and dried in vacuum to constant weight to obtain a white powder....
Embodiment 2
[0027] Example two, the photopolymerization of PEGMA monomer under p-methoxybenzaldehyde / 1-iodoperfluorohexane initiation system
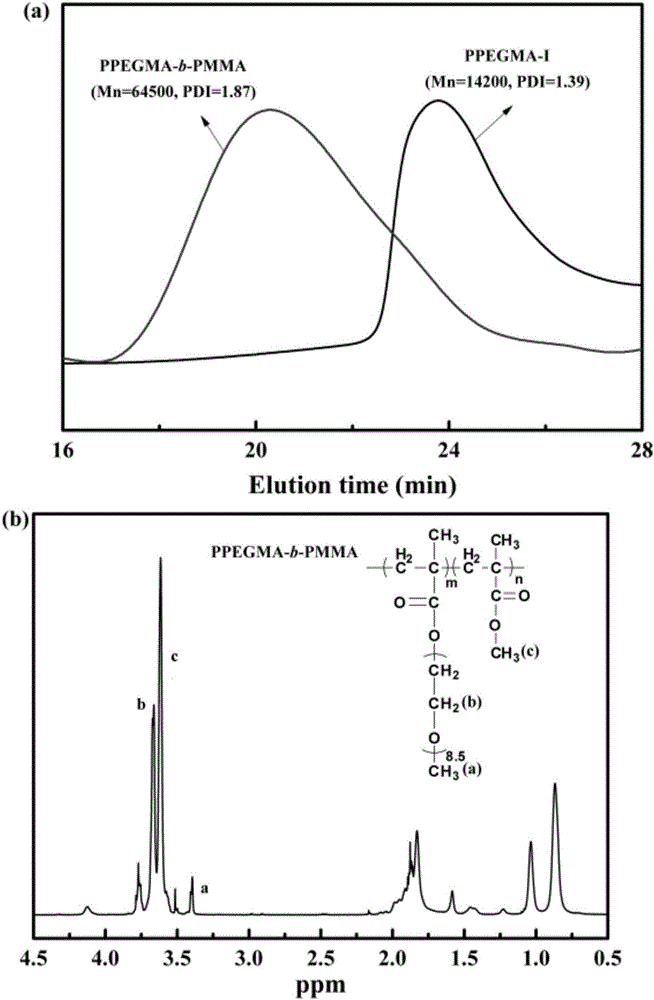
[0028] Referring to Example 1, the photopolymerization of PEGMA monomer under the p-methoxybenzaldehyde / 1-iodoperfluorohexane initiation system was carried out. The molar ratio [PEGMA] / [1-iodoperfluorohexane] / [p-methoxybenzaldehyde] / [DMA] was 42 / 2 / 5 / 5. Samples taken at different time periods were precipitated with ether three times, dried in vacuum to constant weight, and a viscous substance was obtained. At 20.5h, the monomer conversion rate was 88%, and the polymer product M was recorded by GPC. n= 14200, PDI = 1.39. In addition, during the polymerization process, the molecular weight of the polymer increases linearly with the increase of the conversion rate, and the molecular weight distribution of the polymer is narrow (less than 1.49), which conforms to the law of living polymerization.
Embodiment 3
[0029] Embodiment three, the photopolymerization of MMA monomer under p-methoxybenzaldehyde / other initiator initiation system
[0030] Referring to Example 1, carry out the photopolymerization of the MMA monomer under p-methoxybenzaldehyde / other initiator initiation system. The difference is that the selected initiators are ethyl 2-bromoisobutyrate, ethyl α-bromophenylacetate, 1-iodoperfluorobutane and 1-iodoperfluorooctane, etc.
[0031] Specifically, the monomer concentration is 33 wt%, and the molar ratio [MMA] / [ethyl 2-bromoisobutyrate] / [p-methoxybenzaldehyde] / [DMA]=100 / 1 / 10 / 50 The substance was added into the DMF solvent, reacted for 36h, and the monomer conversion rate was 85%, and the polymer product M was recorded by GPC. n = 26100, PDI = 1.72. Under the same conditions, when the molar ratio [MMA] / [α-ethyl bromophenylacetate] / [p-methoxybenzaldehyde] / [DMA]=100 / 1 / 10 / 50, the reaction was 35h, and the monomer The conversion rate was 90%, and the polymer product M was re...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Monomer conversion | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com