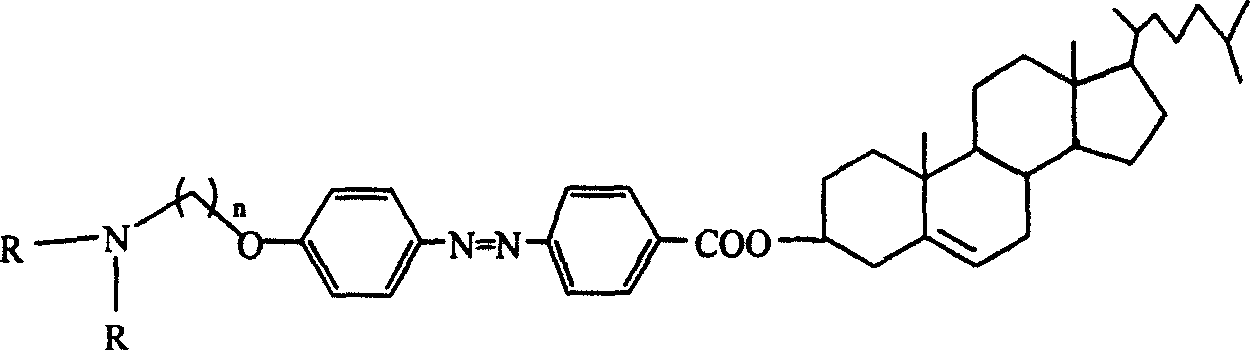
Cholesterol derivative containing azobenzene group, and its synthesizing method and use
A technology of cholesterol derivatives and azophenyl, which is applied in medical preparations of non-active ingredients, steroids, organic chemistry, etc., can solve problems such as poor stability, and achieve the effect of easy operation and less harsh reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
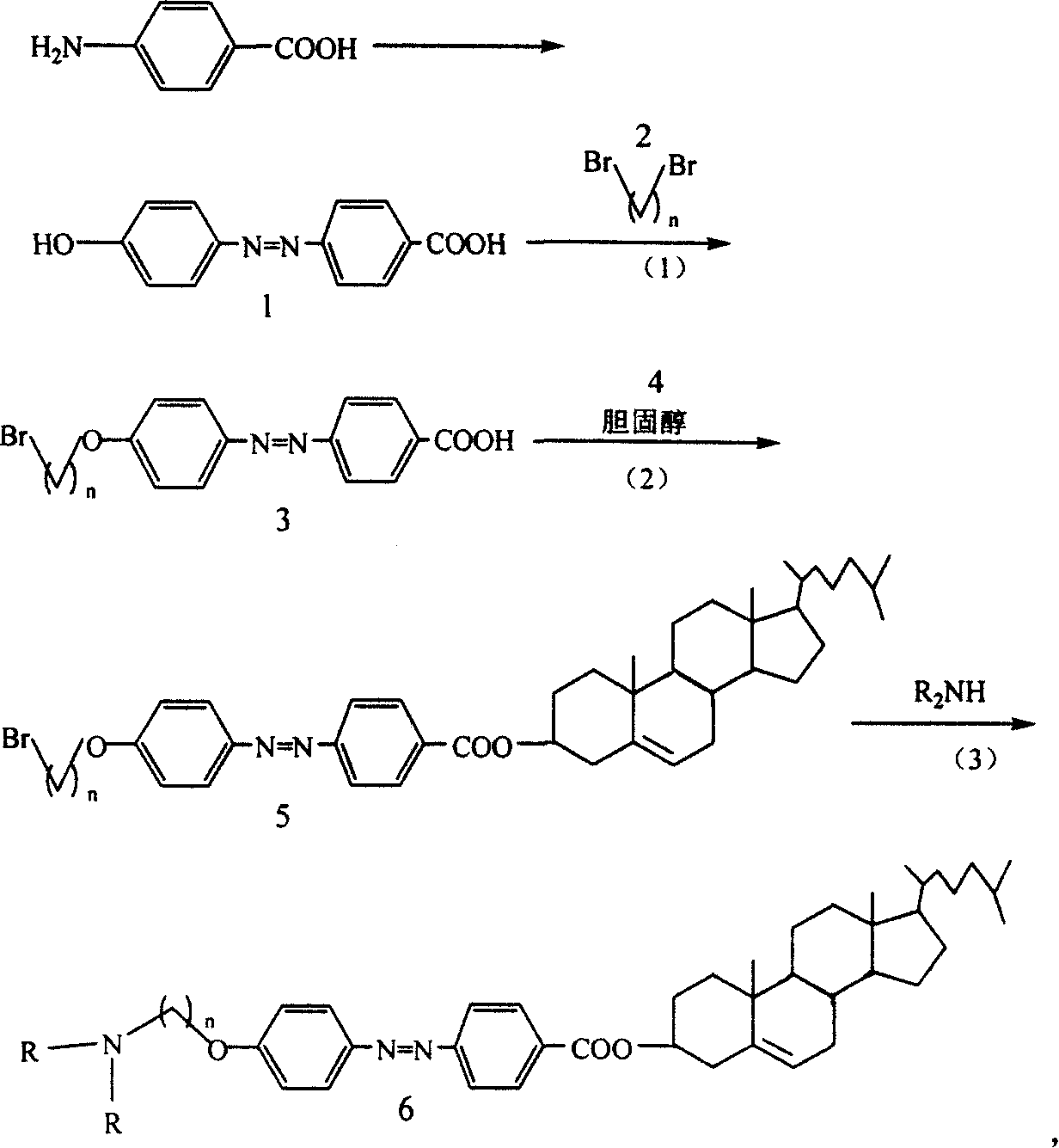
[0024] Dissolve 8mmol of compound 1 in 200ml of anhydrous acetone, add 18mmol of anhydrous K 2 CO 3 , 2mmol KI or 18-crown-6, and 32mmol compound 2 were refluxed for 36h and filtered, the filtrate was spin-dried and separated by flash column chromatography to obtain compound 3 (90%).
[0025] C 17 h 17 N 2 o 3 Br
[0026] 1 H NMR (300MHz, DMSO, ppm): 8.14-8.11 (2H, d, J=9Hz), 7.94-7.90 (4H, dd, J=6Hz), 7.17-7.14 (2H, d, J=9Hz), 4.16 -4.12(2H, t, 6Hz), 3.65-3.61(2H, t, 6Hz), 2.02-1.86(4H, m).
[0027] MS(EI): 424(M + +47, 100%), 378 (M + +1, 49.16%), 376 (M + -1, 49.71%), 183 (M + -194, 56.65%), 133 (M + -244, 28.83%), 121 (M + -244, 25.66).
[0028] IR: 1681, 1602, 1581, 1501, 1501, 1427, 1277, 1248, 1142.
Embodiment 2
[0030] Add 6mmol of compound 3 to 100ml of dichloromethane, and then dropwise add 50ml of a dichloromethane solution containing 7mmol of dicyclohexylcarbodiimide. After the solution is clarified, add dropwise 50ml of a dichloromethane solution containing 7mmol of compound 4, and react at room temperature for 12h. Filter, extract with 0.1N hydrochloric acid, then wash with saturated NaHCO 3 Extraction with aqueous solution, and finally extraction with bromine water, the organic phase was spin-dried, and separated by flash column chromatography to obtain compound 5 (55%).
[0031] C 44 h 61 N 2 o 3 Br
[0032] 1 H NMR (300MHz, CDCl 3, ppm): 8.18-8.15 (2H, d, J = 9Hz), 7.96-7.88 (4H, dd, J = 9Hz), 7.02-6.99 (2H, d, J = 9Hz), 5.44-5.42 (1H, m ), 4.94-4.83(1H, m), 4.11-4.07(2H, t, J=6Hz), 3.53-3.49(2H, t, J=6Hz), 2.50-0.69(47H, m).
[0033] MS (MALDI): 745.4 (M + ), 747.4 (M + +2).
[0034] IR: 2939, 1722, 1708, 1602, 1500, 1468, 1282, 1257, 1143, 1116.
Embodiment 3
[0036] 3 mmol of compound 5a and 5 ml of diethylamine were refluxed in chloroform for 3 days, filtered after cooling, washed with chloroform, and separated by flash column chromatography to obtain compound 6a (50%). where n=4.
[0037] C 48 h 71 N 3 o 3
[0038] 1 HNMR (300MHz, CDCl 3 , ppm): 8.19-8.16 (2H, d, J = 9Hz), 7.97-7.89 (4H, dd, J = 9Hz), 7.02-6.99 (2H, d, J = 9Hz), 5.44 (1H, m), 4.93-4.83(1H, m), 4.11-4.07(2H, t, J=6Hz), 3.23-3.16(6H, m), 2.51-0.69(53H, m)
[0039] MS (MALDI): 737 (M + ), 738 (M + +1), 739(M + +2)
[0040] IR: 2926, 2854, 1713, 1601, 1583, 1502cm -1
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com