Human source anti- tetanus exotoxin antibody and preparation method and use thereof
An anti-tetanus and exotoxin technology, applied in the fields of botanical equipment and methods, biochemical equipment and methods, antibodies, etc., can solve problems such as insufficient blood supply
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
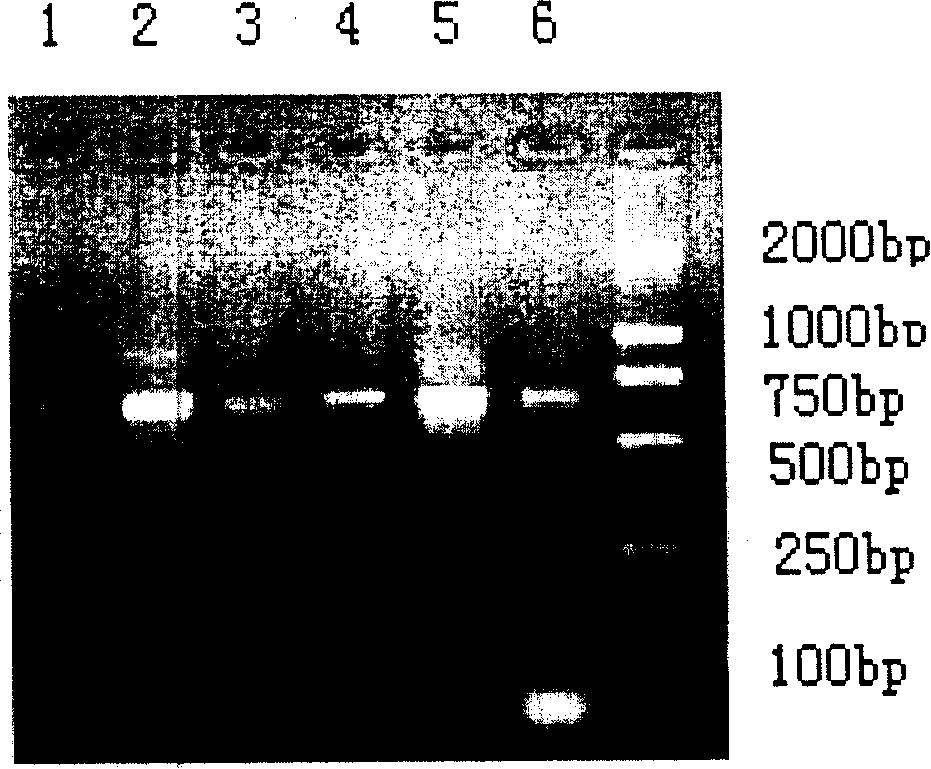
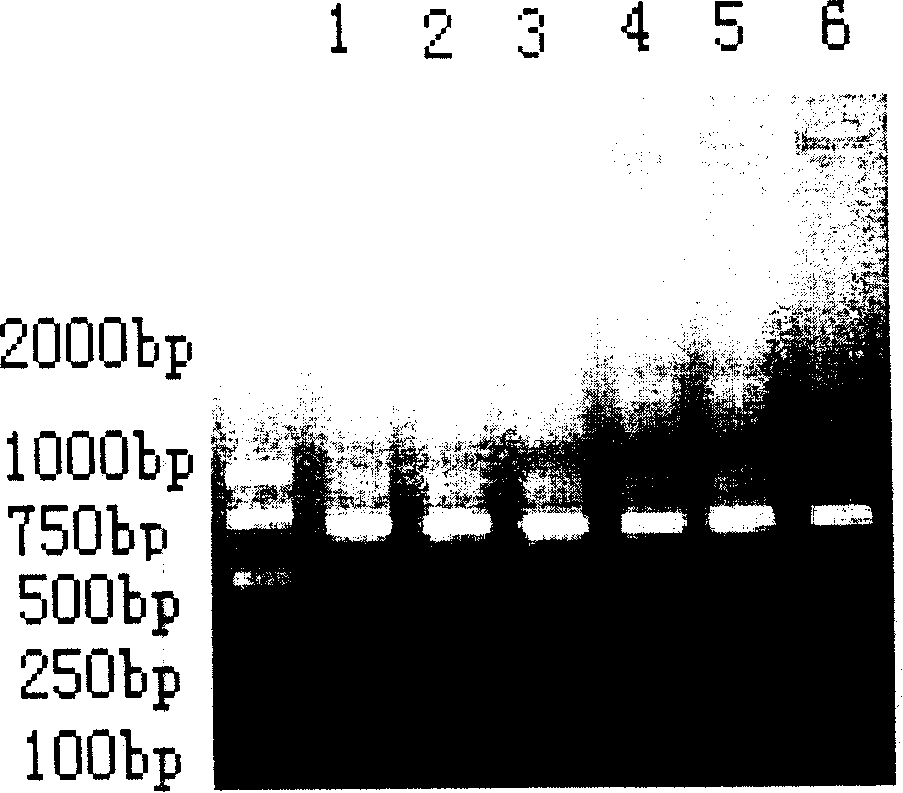
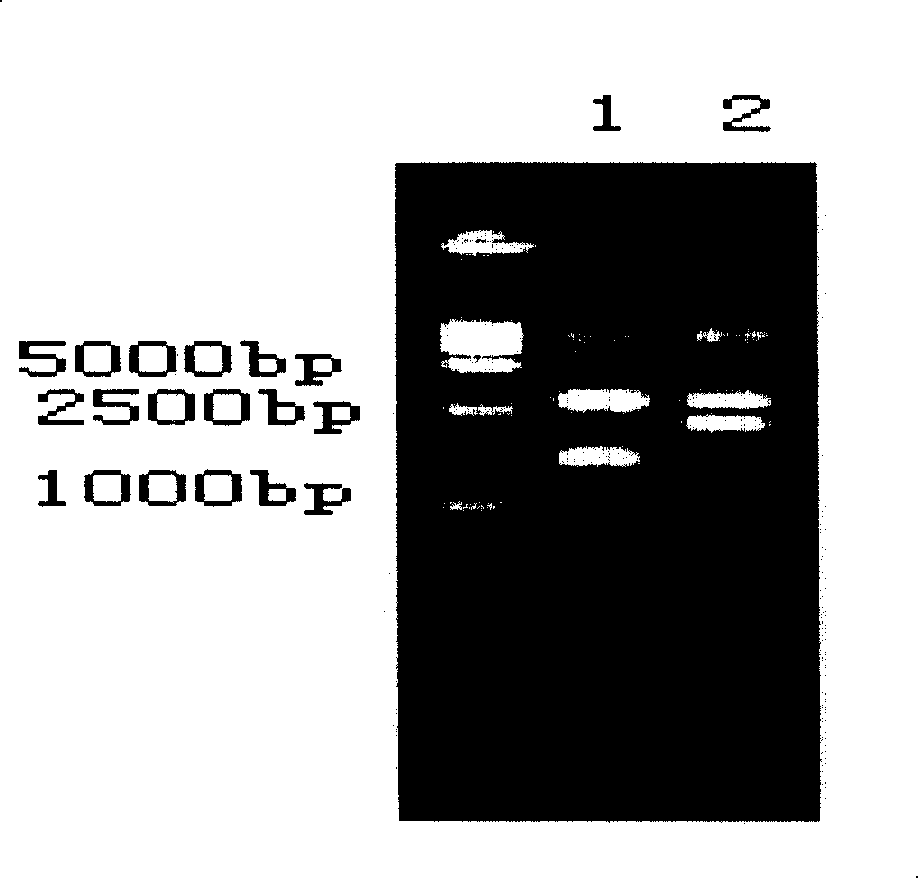
[0110] Phagemid pComb 3 (4000bp) was produced by Scripps Research Institute of the United States; XL1-Blue and helper phage VCSM13 were purchased from Stratagene Company of the United States; Trizol was purchased from Gibco-BRL Company; PCR kits, restriction enzymes, T4DNA ligase Purchased from Promega; tetanus exotoxin and standard human tetanus immunoglobulin (TIG) were purchased from China National Institute for the Control of Pharmaceutical and Biological Products. Goat anti-human IgG Fab, inducer IPTG (isopropyl-β-d-thiogalactoside), chromogen OPD (o-phenylenediamine), and 3-morpholine propanesulfonic acid (MOPS) were all from Sigma product. Yeast and CHO were purchased from Promega.
[0111] 1. PCR amplification of human IgG Fab segment gene:
[0112] Lymphocytes were isolated from the anticoagulated blood of volunteers immunized with tetanus toxoid for 6 days with lymphocyte separation medium, total cellular RNA was extracted with Trizol, and the extracted RNA was rev...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com