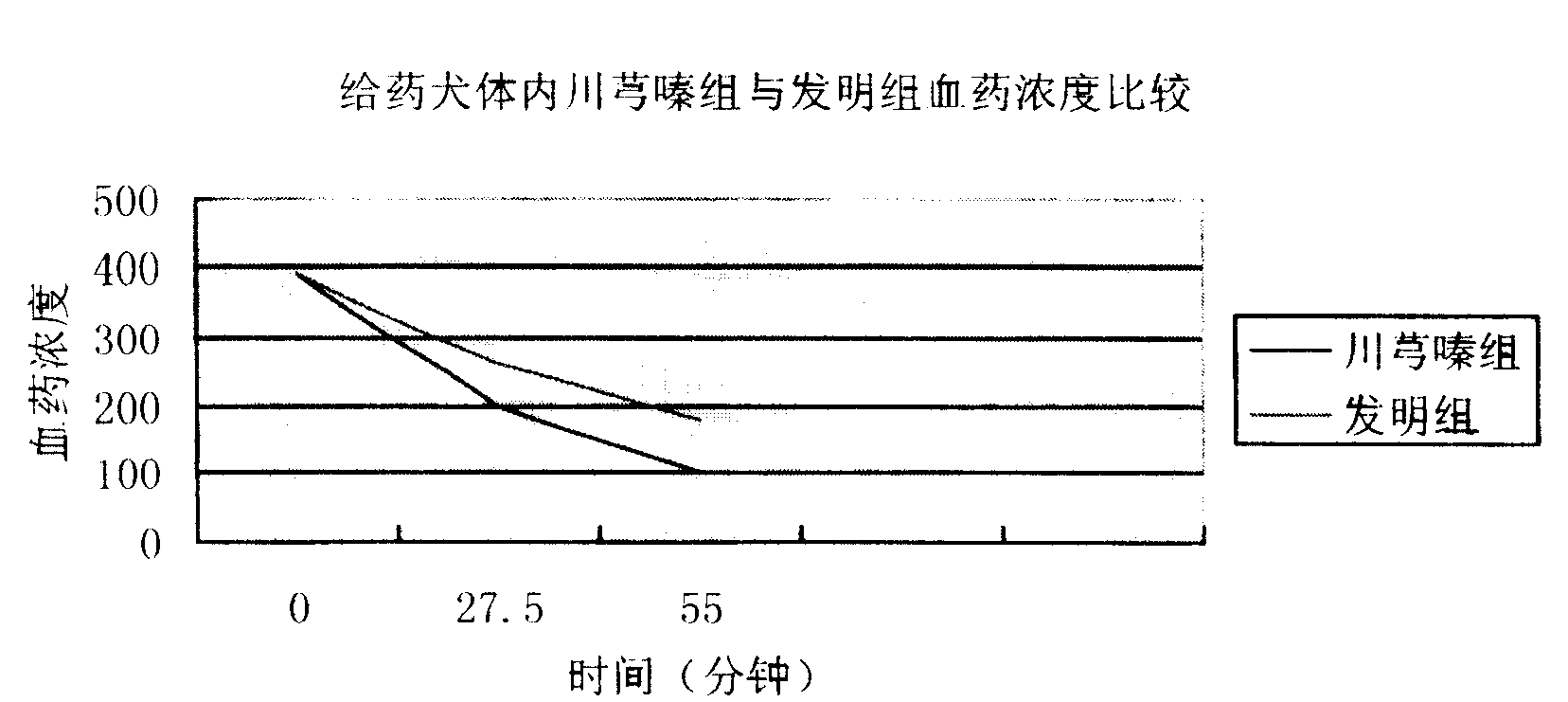
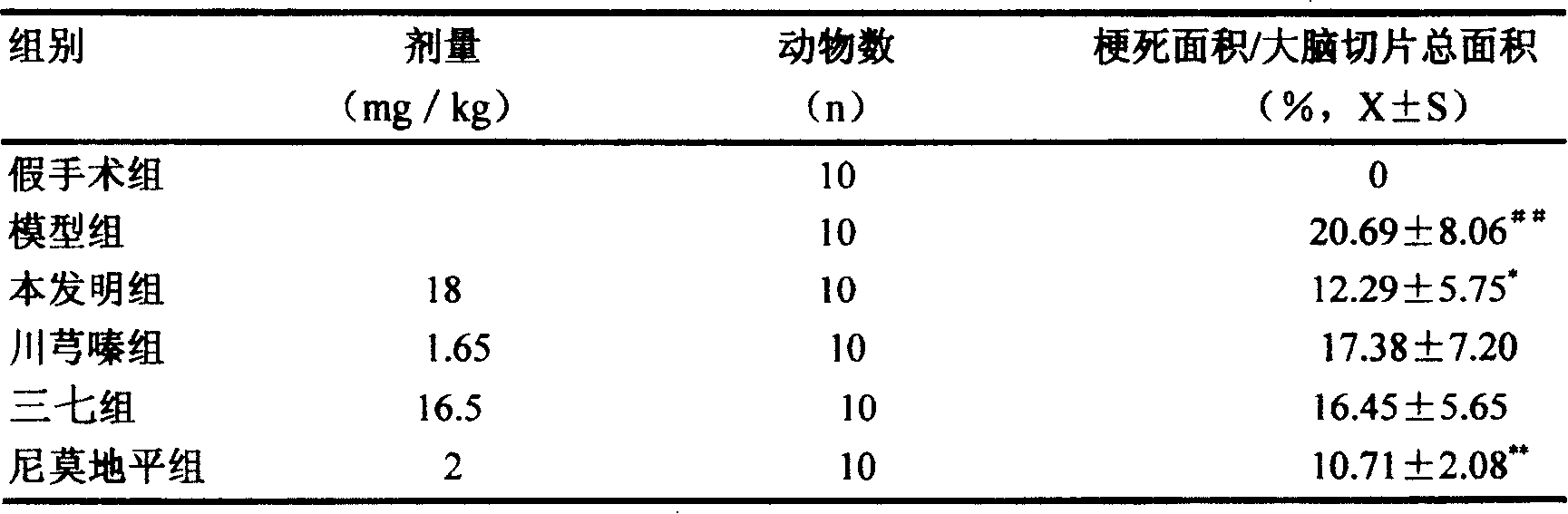
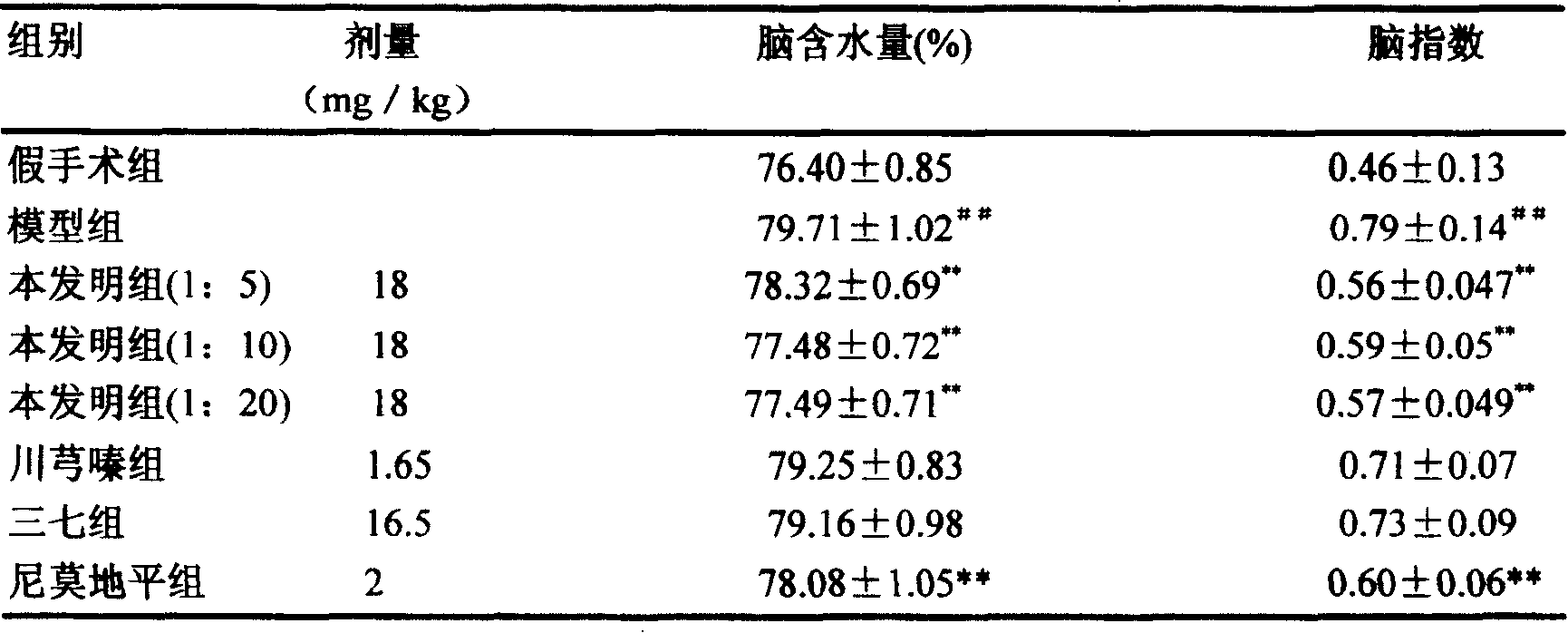
Compound injection from Chunxiongqin and Sanchi general saponin and its preparation
A technology of Panax notoginseng saponins and injection preparations, which is applied in the field of compound injections and its preparation, which can solve the problems of large dosage of saponins and easy occurrence of saponin hemolysis, etc., and achieve the effects of less toxic and side effects, good clinical treatment effects, and abundant resources
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Embodiment 1: the preparation of injection
[0050] Take Ligustrazine 20g, grind finely, add 1000ml water for injection to dissolve completely. Get the raw material of Panax notoginseng total saponins (containing ginsenoside Rg 1 , Rb 1 , notoginsenoside R 1 Total amount 50-90%) 200g add 2-4L water for injection to dissolve. Slowly add the aqueous solution of ligustrazine to the aqueous solution of Panax notoginseng saponins, stir until clear while adding, and dilute to 10L with water for injection. The medicinal liquid is ultrafiltered through an ultrafiltration membrane with a pore size of 10,000 molecular weight, potted, 10 ml / bottle, and sterilized to obtain an injection.
Embodiment 2
[0051] Embodiment 2: the preparation of transfusion
[0052] Take Ligustrazine 13g, grind finely, add 2400ml water for injection and stir to dissolve completely. Get the raw material of Panax notoginseng total saponins (containing ginsenoside Rg 1 , Rb 1 , notoginsenoside R 1 Total amount 50-90%) 200g add 2-4L water for injection to dissolve. Slowly add the aqueous solution of Ligustrazine to the aqueous solution of Panax notoginseng saponins, stir while adding, and add to an isotonic pressure regulator, the isotonic agent is at least one of mannitol, sodium chloride or glucose, And dilute to 100L or 250L with water for injection, stir well. The medicinal solution is ultrafiltered by an ultrafiltration membrane with a pore size of 30,000 molecular weight, supplemented with an appropriate amount of water for injection, subpackaged into 100ml or 250ml / bottle, filled with caps, and sterilized to obtain an infusion solution.
Embodiment 3
[0053] Embodiment 3: the preparation of freeze-dried powder injection
[0054] Take Ligustrazine 20g, grind finely, add 40ml water for injection to dissolve, set aside. Get the raw material of Panax notoginseng total saponins (containing ginsenoside Rg 1 , Rb 1 , notoginsenoside R 1 50-90% of the total amount) 200g, add appropriate amount of water for injection to dissolve. Slowly add the ligustrazine solution into the aqueous solution of Panax notoginseng saponins, stir until clear while adding, dilute to 50L with water for injection, and dissolve. The medicinal solution is ultrafiltered by an ultrafiltration membrane with a flux of 10,000 molecular weight, divided into 5 ml bottles, freeze-dried, capped, and labeled to obtain powder injections, with a total of 10,000 bottles.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com