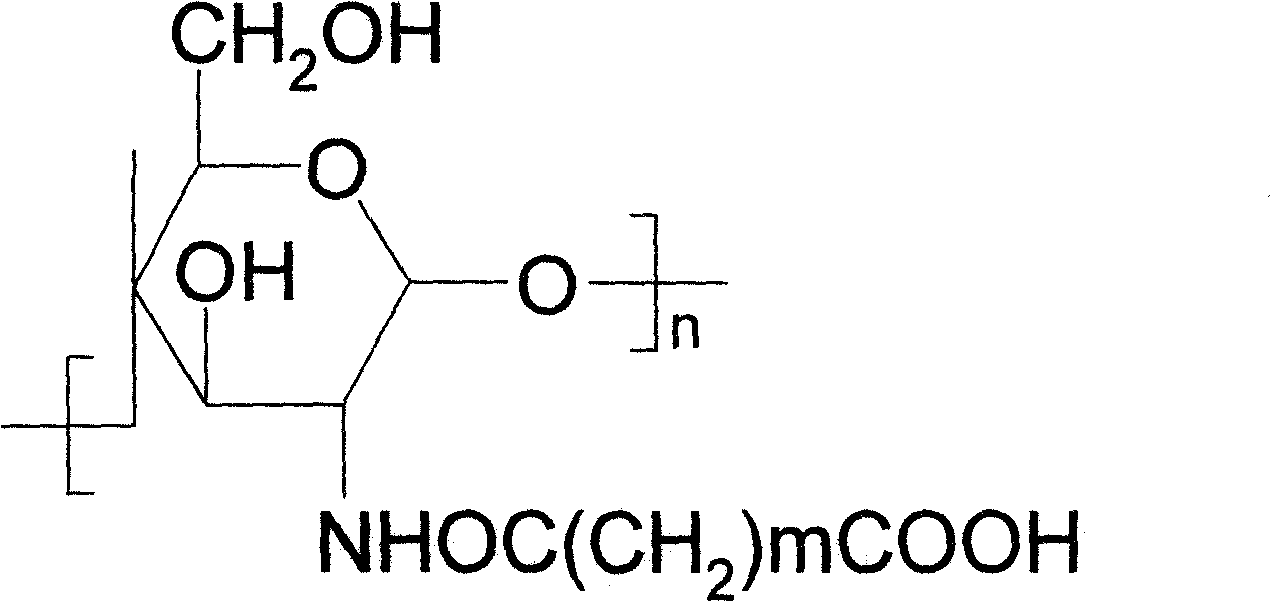
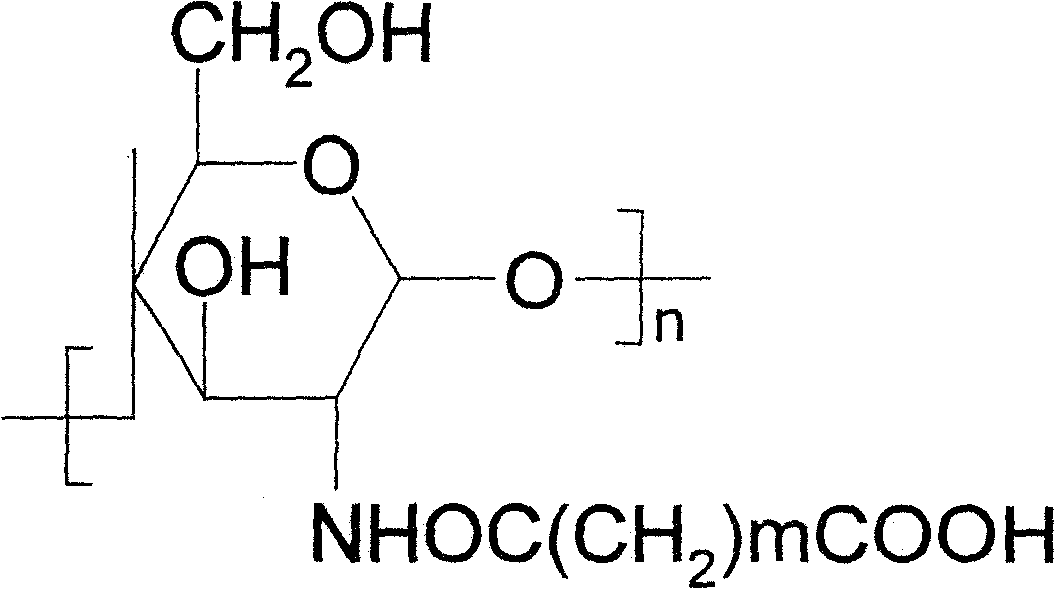
Fixed trypsinase and its preparation method
A trypsin and immobilized enzyme technology, which is applied in the field of bioengineering to achieve the effects of high catalytic efficiency, good production and application value, and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Embodiment 1: determination of activity recovery rate
[0026] A. Take 3g of refined dextran derivative powder, place in 40ml of absolute ethanol solution, stir and heat for 5min, then add 30% acetic anhydride polar organic solution, and heat at 65°C for 6h under reflux. After heating, the precipitate was collected by centrifugation, washed with 50% ethanol until the pH was near neutral, and dried at 60°C to obtain the carrier.
[0027] B. Take 1g carrier, dissolve it in 20ml of phosphate buffer solution with a concentration of 200mmol / L and a pH of 6-9, stir at 10°C for 10min, add 10ml of a cross-linking agent solution with a concentration of 10% and 10ml of a cross-linking agent solution with a concentration of 100mg / ml trypsin solution (enzyme activity: 3000U), stirred at 10°C for 4h. After the reaction, adjust the pH of the solution until the immobilized enzyme is completely precipitated, and collect the precipitate by centrifugation to obtain the immobilized tryps...
Embodiment 2
[0030] Embodiment 2: optimum pH and optimum temperature determination
[0031] A. Take 4 g of refined dextran derivative powder, put it in 50 ml of acetone, stir and heat for 5 min, then add a polar organic solution of malonic anhydride with a concentration of 20%, and heat under reflux at 70° C. for 4 h. After heating, the precipitate was collected by centrifugation, washed with 30% ethanol until the pH was near neutral, and dried at 70°C to obtain the carrier.
[0032] B, get 1g carrier, be dissolved in the aforementioned phosphate buffer of 20ml, after stirring 10min at 15 DEG C, add 10ml concentration to the solution and be the trypsin solution that 10% cross-linking agent solution and 10ml concentration are 100mg / ml (enzyme activity is 3000U), stirred at 15°C for 6h. After the reaction is completed, the pH of the solution is adjusted until the immobilized enzyme is completely precipitated, and the precipitate is collected by centrifugation to obtain the immobilized tryps...
Embodiment 3
[0035] Embodiment 3: Determination of acid-base solubility
[0036] A. Take 20 g of refined dextran derivative powder, place it in 500 ml of dimethyl sulfoxide solvent, stir and heat for 5 min, then add 10% succinic anhydride polar organic solution, and heat at 65° C. for 8 h under reflux. After heating, the precipitate was collected by centrifugation and washed with 30% ethanol until the pH was near neutral. Dry at 70°C to obtain the carrier.
[0037] B. Get 10g of carrier, dissolve it in 200ml of buffer, stir at 13°C for 10min, add 100ml of cross-linking agent solution with a concentration of 10% and 100ml of trypsin solution with a concentration of 100mg / ml (enzyme activity is 3000U) to the solution , Stir at 13°C for 4h. After the reaction, adjust the pH of the solution until the immobilized enzyme is completely precipitated, and collect the precipitate by centrifugation to obtain the immobilized trypsin.
[0038] C. Take 10 g of immobilized trypsin, add it to 300 ml of...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com