New crystalline form iii of agomelatine, a process for its preparation and pharmaceutical compositions containing it
A technology of crystal form and medicine, applied in the new crystal form III of N-[2-ethyl]acetamide: field, can solve problems such as no specific description
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
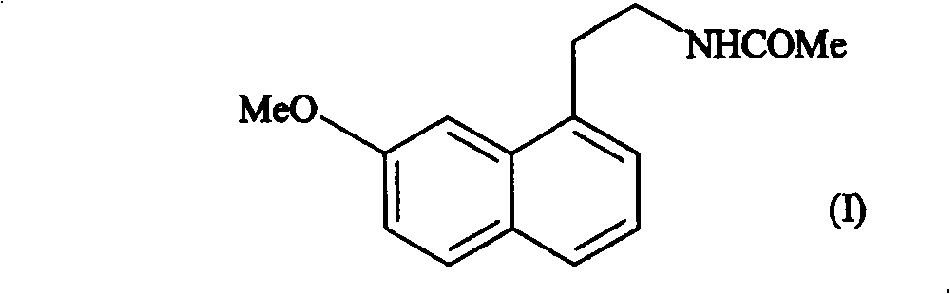
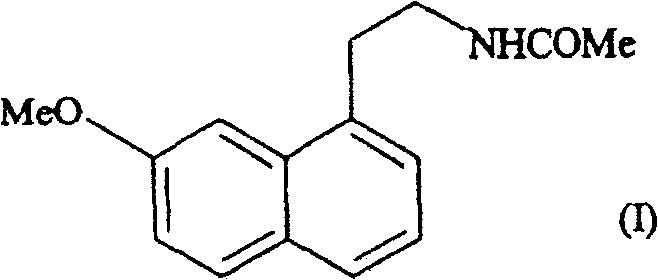
[0018] Example 1: Form III of N-[2-(7-methoxy-1-naphthyl)ethyl]acetamide
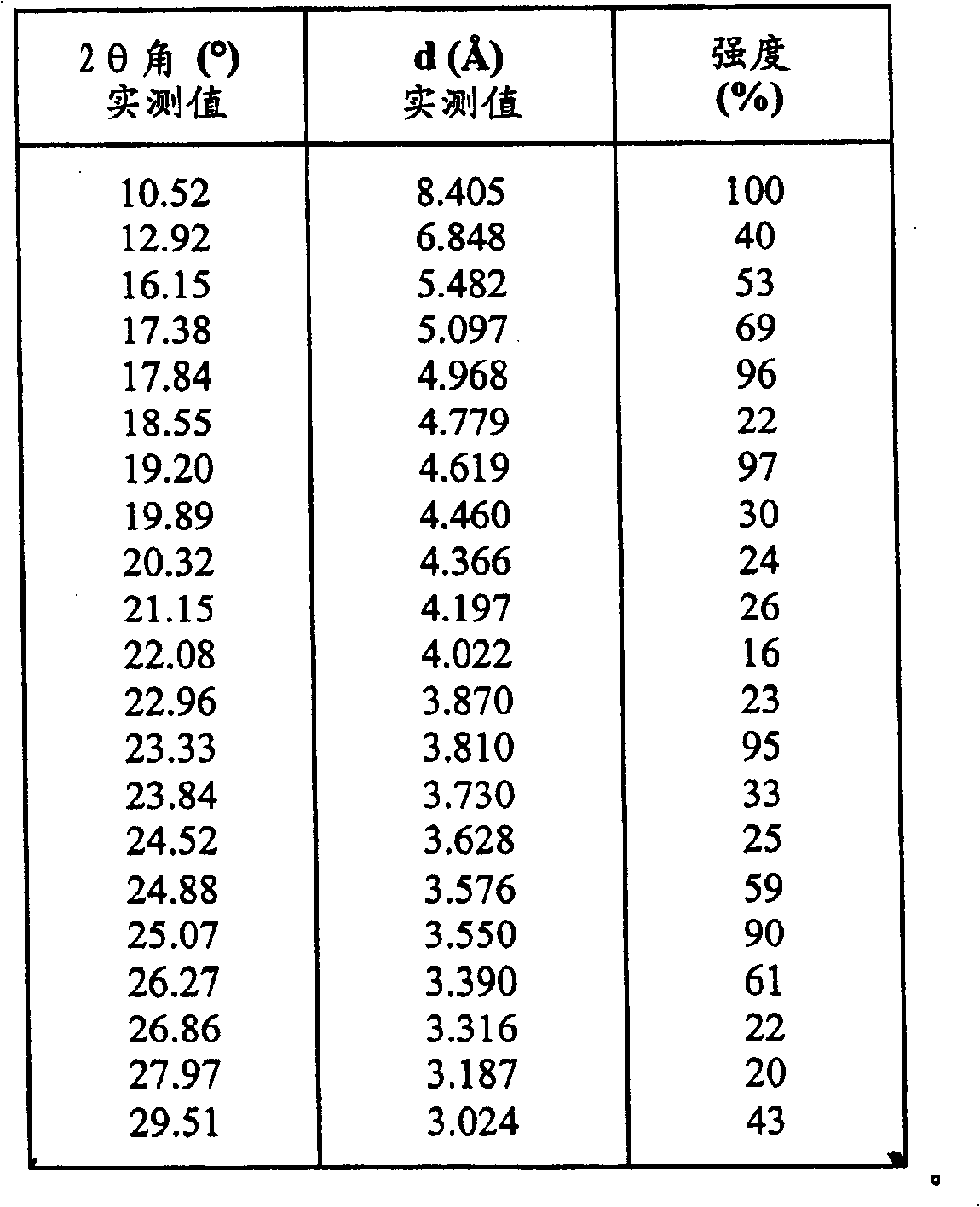
[0019] Heat 100 g of N-[2-(7-methoxy-1-naphthyl)ethyl]acetamide in a ventilated incubator at 110°C until it melts completely, then cool slowly until crystallization. The obtained crystalline form III is characterized by the following powder X-ray diffraction pattern, which is measured using a Siemens D5005 diffractometer (copper to cathode), expressed in terms of interplanar distance d, Bragg 2θ angle, intensity and relative intensity (expressed as a percentage of the strongest ray) :
[0020]
Embodiment 2
[0021] Embodiment 2: pharmaceutical composition
[0022] Prepare a prescription for 1000 tablets, each containing a 25mg dose:
[0023] Compound of Example 1 ................................................ ......25g
[0024] Lactose monohydrate ................................................ ..........62g
[0025] Magnesium stearate.............................................. ..........1.3g
[0026] corn starch................................................ ..........26g
[0027] Maltodextrin mixture................................................ ......9g
[0028] Anhydrous colloidal silica................................................ ......0.3g
[0029] Sodium Starch Glycolate Type A ................................................ .......4g
[0030] Stearic acid................................................ ..........2.6g
Embodiment 3
[0031] Embodiment 3: pharmaceutical composition
[0032] Prepare a prescription for 1000 tablets, each containing a 25mg dose:
[0033] Compound of Example 1 ................................................ ......25g
[0034] Lactose monohydrate ................................................ ..........62g
[0035] Magnesium stearate.............................................. ..........1.3g
[0036] Povidone................................................ ................9g
[0037] Anhydrous colloidal silica................................................ ......0.3g
[0038] Sodium carboxymethyl cellulose............................................ ..........30g
[0039] Stearic acid................................................ ..........2.6g
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com