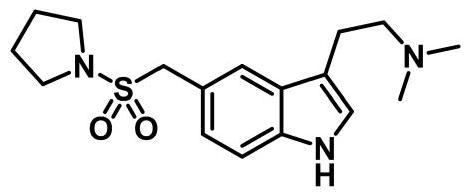
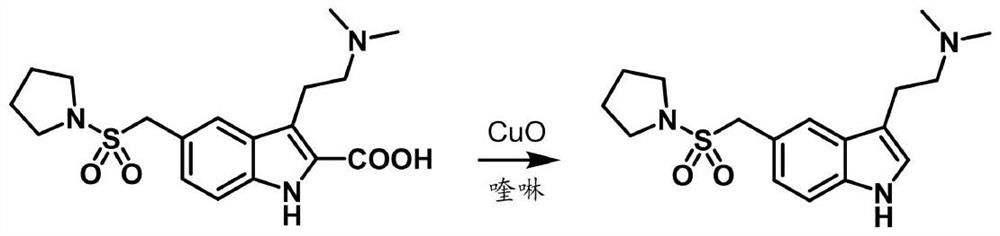
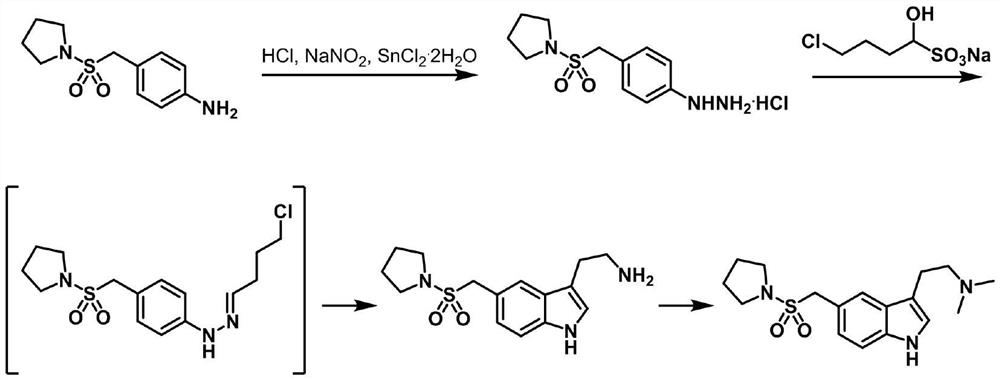
Preparation method of anti-migraine drug almotriptan
A technology for almotriptan and migraine, which is applied in the field of preparing an anti-migraine drug almotriptan, can solve the problems of high cost of ligands, small feeding amount, large dosage and the like, and achieves convenient purification, high overall yield, The effect of simple operation in the preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Preparation of ethyl 2-(5-((pyrrolidinyl-1-sulfonyl)methyl)-1-p-toluenesulfonyl-1H-indolyl-3-) ethyl acetate formula (III)
[0047] Under nitrogen protection, charged with N-(2-iodo-4-((pyrrolidinyl-1-sulfonyl)methyl)phenyl)-4-methylbenzenesulfonamide (formula (I) (26 g, 50 mmol) ), ethyl 4-acetoxycrotonate formula (II) (8.6g, 50mmol), palladium acetate (0.23g, 1mmol), tris(o-methylphenyl)phosphine (0.6g, 2mmol), diisopropyl Anhydrous N,N-dimethylacetamide (125ml) was added to a 250ml three-necked flask of ethyl ethylamine (15.5g, 0.12mol) and a rotor, stirred overnight in an oil bath at 100°C, and cooled to room temperature after the reaction was completed. , add water (25ml), let stand under ice bath for 2h, filter, wash the filter cake with water (50ml) and ethanol (50ml) successively, beat with ethyl acetate to obtain a white solid (formula (III)) (19g, 38mmol), collect The rate is 76%.
[0048] H 1 NMR (400MHz, DMSO): 7.92(d,1H), 7.84(d,2H), 7.76(s,1H), 7.56(s,1...
Embodiment 2
[0050] Preparation of ethyl 2-(5-((pyrrolidinyl-1-sulfonyl)methyl)-1-p-toluenesulfonyl-1H-indolyl-3-) ethyl acetate formula (III)
[0051] Under nitrogen protection, charged with N-(2-bromo-4-((pyrrolidinyl-1-sulfonyl)methyl)phenyl)-4-methylbenzenesulfonamide (formula (I)) (2.4 g , 5mmol), ethyl 4-acetoxycrotonate (formula (II)) (860mg, 5mmol), palladium acetate (45mg, 0.2mmol), tris(o-methylphenyl)phosphine (122mg, 0.4mmol), Anhydrous N,N-dimethylacetamide (15ml) was added to a 50ml three-necked flask of diisopropylethylamine (1.6g, 12mmol) and a rotor, stirred overnight in an oil bath at 120°C, and cooled after the reaction was completed. After reaching room temperature, additional water (3ml) was added, and it was allowed to stand for 2h in an ice bath, filtered, and the filter cake was washed with water (6ml) and ethanol (6ml) in turn, and ethyl acetate was slurried to obtain a white solid (formula (III)) (2.07g, 4.1 mmol), the yield was 80%.
Embodiment 3
[0053] Preparation of ethyl 2-(5-((pyrrolidinyl-1-sulfonyl)methyl)-1-p-toluenesulfonyl-1H-indolyl-3-)acetate formula (III) Loaded with N-(2-iodo-4-((pyrrolidinyl-1-sulfonyl)methyl)phenyl)-4-methylbenzenesulfonamide (formula (I)) (2.6 g, 5 mmol), 4 - Ethyl acetoxycrotonate (formula (II)) (860 mg, 5 mmol), tris(dibenzylideneacetone)dipalladium) (183 mg, 0.2 mmol), tris(o-methylphenyl)phosphine (122 mg, 0.4mmol), diisopropylethylamine (1.6g, 12mmol) and a 50ml three-necked flask of the rotor were added anhydrous N,N-dimethylacetamide (15ml), stirred overnight in an oil bath at 100°C, After the reaction was completed, it was cooled to room temperature, water (3ml) was added, left standing for 2h in an ice bath, filtered, the filter cake was washed with water (6ml) and ethanol (6ml) successively, and ethyl acetate was beaten to obtain a white solid (formula (III)) ( 1.96 g, 3.9 mmol) in 78% yield.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com