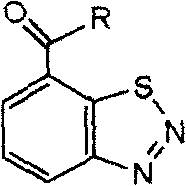
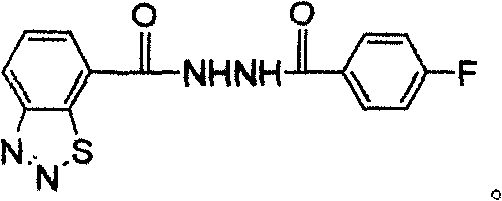
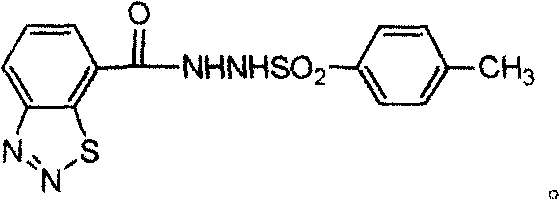
Diazosulfide derivative and its synthesis and screening method for inducing anti-disease activity
A technology of benzothiadiazole and derivatives, applied in the field of benzothiadiazole derivatives
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0075] Synthesis of 3-amino-2-mercaptobenzoic acid:
[0076] In a 250mL three-necked flask, install a reflux condenser, a thermometer and a mechanical stirrer, suspend 13.0g of 62.44mmol of 2-amino-7-methoxycarbonylbenzothiazole in 150mL of ethylene glycol, and add 17.0g of hydrogen peroxide at one time Potassium, heated to 160 ° C for 20 hours, then cooled to room temperature, the reaction mixture was poured into 450 mL of water, adjusted to pH=2 with acetic acid, and the resulting solid was filtered to obtain 6.86 g of the product, with a reaction yield of 64.9%.
Embodiment 2
[0078] Synthesis of benzo[1,2,3]thiadiazole-7-carboxylic acid using intermediate 3-amino-2-mercaptobenzoic acid:
[0079] Install a reflux condenser, a thermometer and a mechanical stirrer in a 500mL three-necked flask, add 33.8g of 3-amino-2-mercaptobenzoic acid and 280mL of 18% dilute hydrochloric acid, control the temperature at -5°C, and slowly add 27.6g of The concentration of sodium nitrite preparation is 40% aqueous solution, the temperature is not more than 0 ℃ when dropping, after dropping, continue to react at low temperature for 1 to 2 hours, wash with water, and filter to obtain 36g of solid initial product.
Embodiment 3
[0081] Preparation of benzo[1,2,3]thiadiazole-7-carbonyl chloride:
[0082] Add 27.0g of benzo[1,2,3]thiadiazole-7-carboxylic acid, 200mL of anhydrous toluene, 1mL of DMF, and 20mL of SOCl in a 500mL three-neck flask 2 , slowly raise the temperature to 80-90°C, keep this temperature and continue to react for 6 hours, filter after cooling, and desolvate the filtrate under reduced pressure to obtain 28g of the product benzo[1,2,3]thiadiazole-7-carbonyl chloride, the product does not need Purified and stored in a desiccator for direct use in the next reaction.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com