Method of synthesizing 9-phenanthrene acid
A synthetic method and technology of phenanthrene, applied in a synthetic field of 9-phenanthrene, can solve the problems of low yield, numerous steps, difficult post-processing and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0035] 1. Synthesis experiment and post-processing:
[0036] In a 25mL three-necked flask, 9-bromophenanthrene (1g, 4mmol) was dissolved in 5mL of ether, and then n-butyllithium (3mL, 8mmol) was added under anhydrous and oxygen-free conditions protected by argon, and stirred for 0.5 hours. During this period, the anhydrous and oxygen-free state was maintained; after that, the reaction solution was poured on dry ice and covered with dry ice; after the dry ice evaporated, 50ml of ether and water (volume ratio 1:1) were added, the water layer was separated, and washed with 50mL of ether 3-5 times to obtain a clear solution. Acidify with 1 mol / L sulfuric acid to pH=2, filter with suction and wash with water to obtain a white solid, dry at 80°C, weigh the product, and the yield is 65%. Its reaction formula is as follows:
[0037]
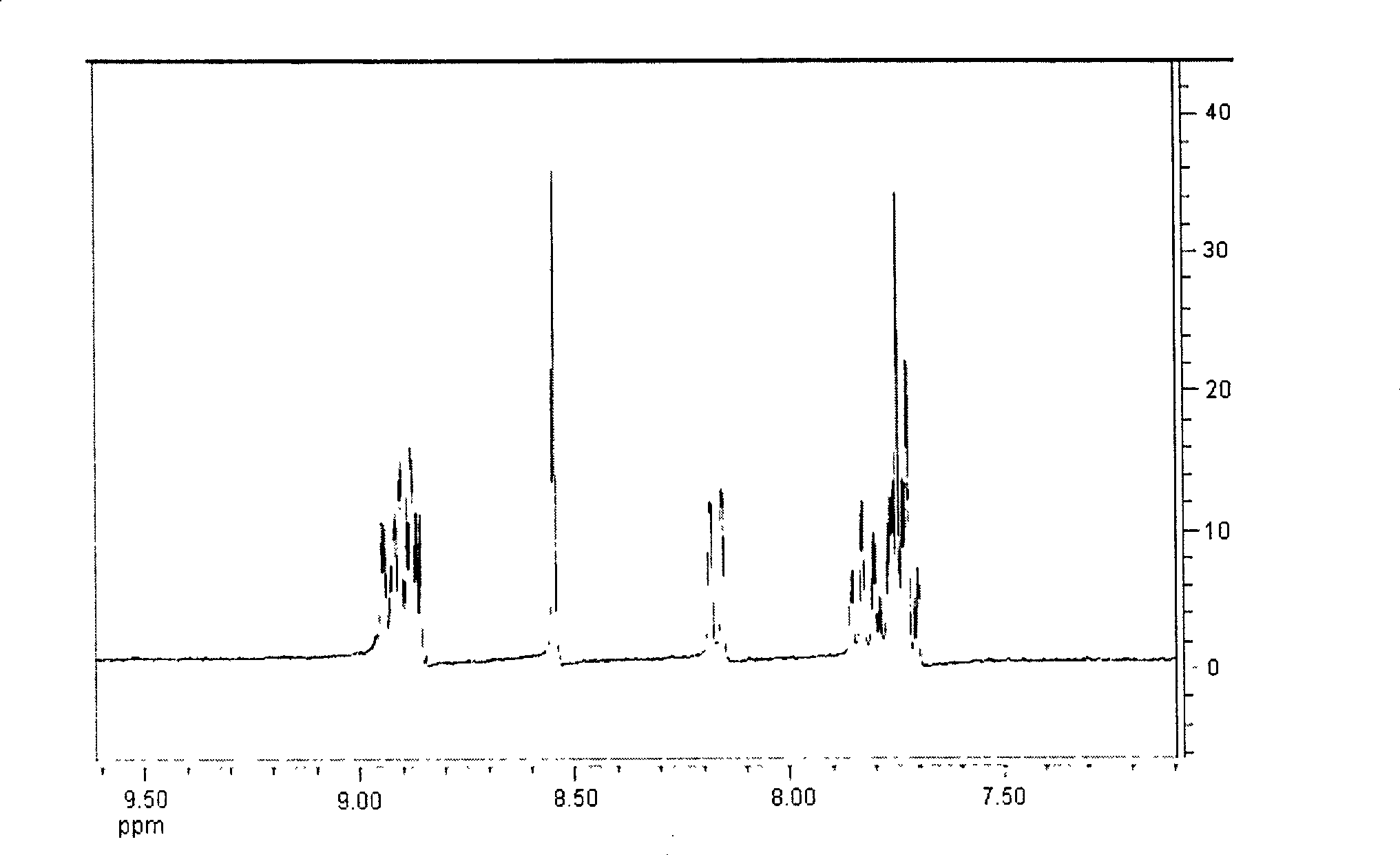
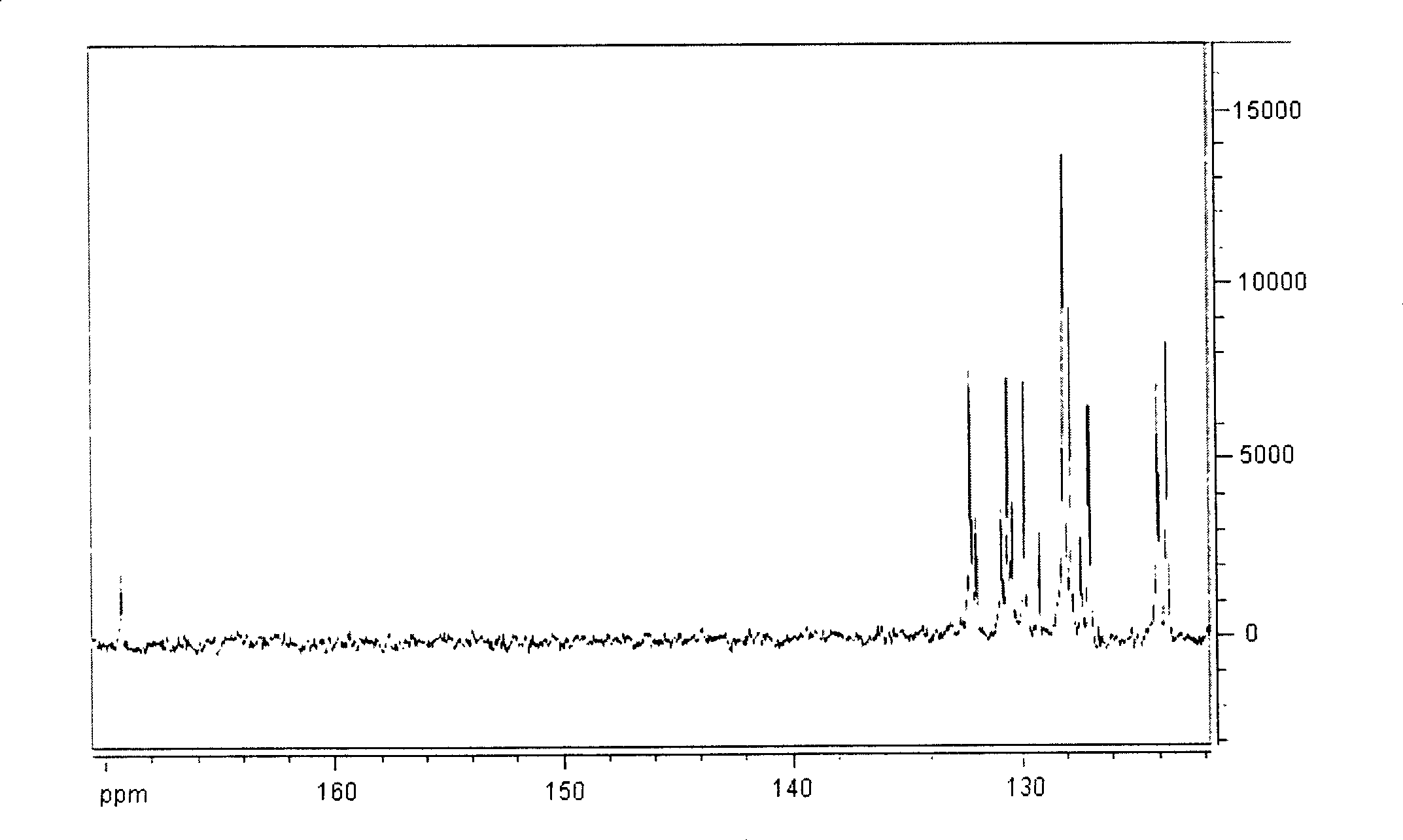
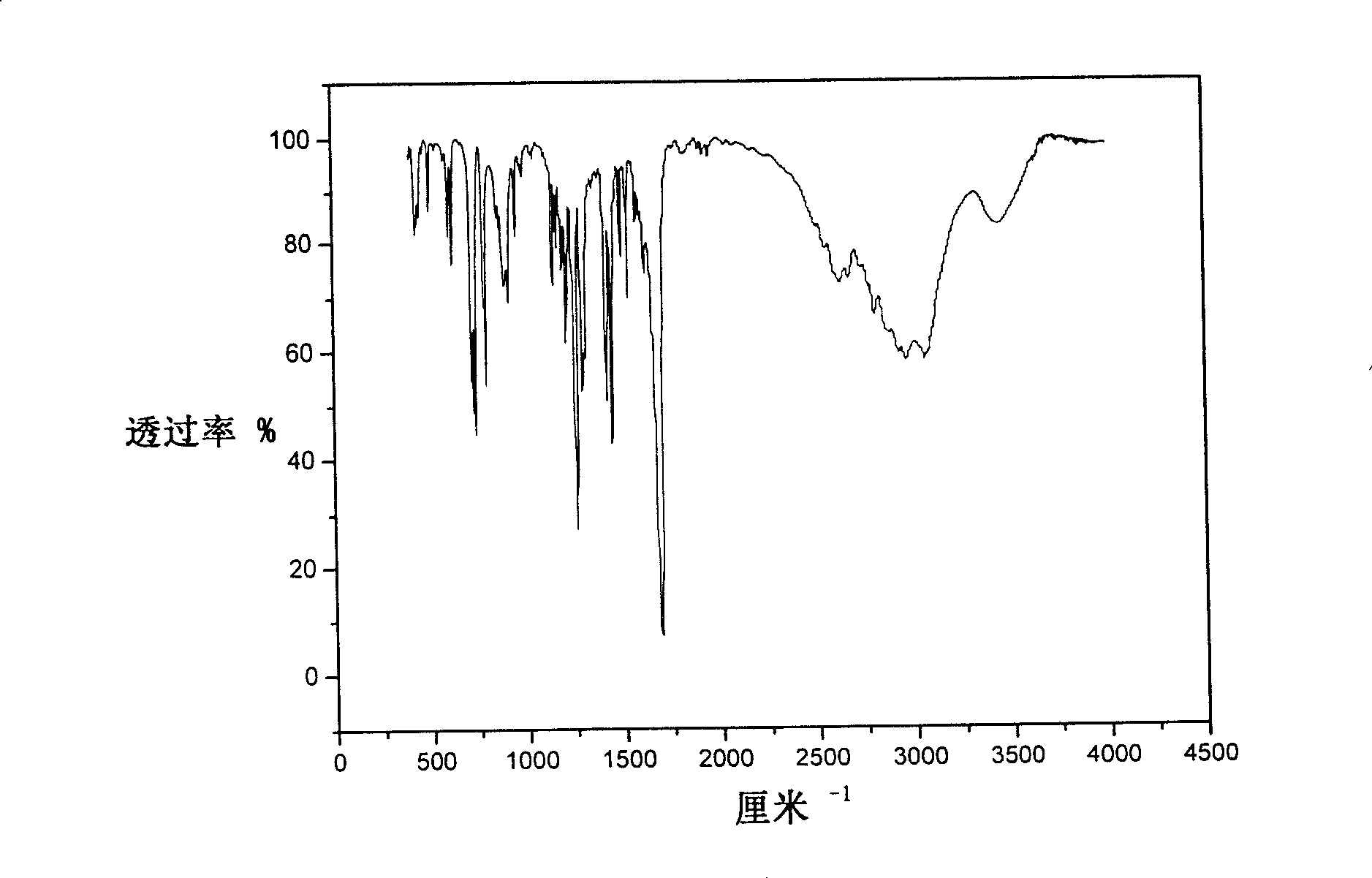
[0038] Elemental analysis: Perkin-Elmer 240 elemental analyzer was used for determination of C and H content; NMR: Bruker AC-P500 NMR (300MHz); Inf...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com