A new type of antibacterial active peptide based on the body's own secretion
A new type of antibacterial activity technology, applied in the field of biochemistry, can solve the problems of consuming a lot of manpower, material resources, large amount of calculation, and insufficient structural diversity, and achieve the effects of low cost, high biological stability, and avoiding immune damage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] The 70-101 AA sequence segment of SPLUNC1 protein was selected as the core region of the active peptide to prepare a new type of antibacterial active peptide AMP1 (NLPLLDILKPGGGTSGGLLGGLLGKVTSVIPG).
[0038] (1) Activated resin: wash and activate Wang's resin with dichloromethane (DCM), stand-by;
[0039] (2) Cross-linking of Fmoc-protected amino acids and resins: Dimethylformamide (DMF), DIEA (N,N-diisopropylethylamine), O-benzotriazole-tetramethylurea hexafluoro Phosphate (HBTU) is mixed with the C-terminal amino acid of the Fmoc-protected target polypeptide; then the mixture is added to the activated resin, and the reaction is shaken to cross-link the Fmoc-protected amino acid with the resin;
[0040] (3) Remove the first Fmoc protecting group and expose the active amino group: use a DMF solution containing 20% piperidine as a deprotecting agent, soak the Fmoc-protected polypeptide 3 fixed on the resin with the deprotecting agent Minutes, drain the resin, filter o...
Embodiment 2
[0048] The 165-191 AA sequence segment of SPLUNC1 protein was selected as the core region of the active peptide to prepare a new type of antibacterial active peptide AMP2 (AVRDKQHRIHLVLGDCTHSPGSLQISL).
[0049] The preparation method and steps of the novel antibacterial active peptide AMP2 are the same as the preparation method and steps of the novel antibacterial active peptide AMP1, refer to Example 1.
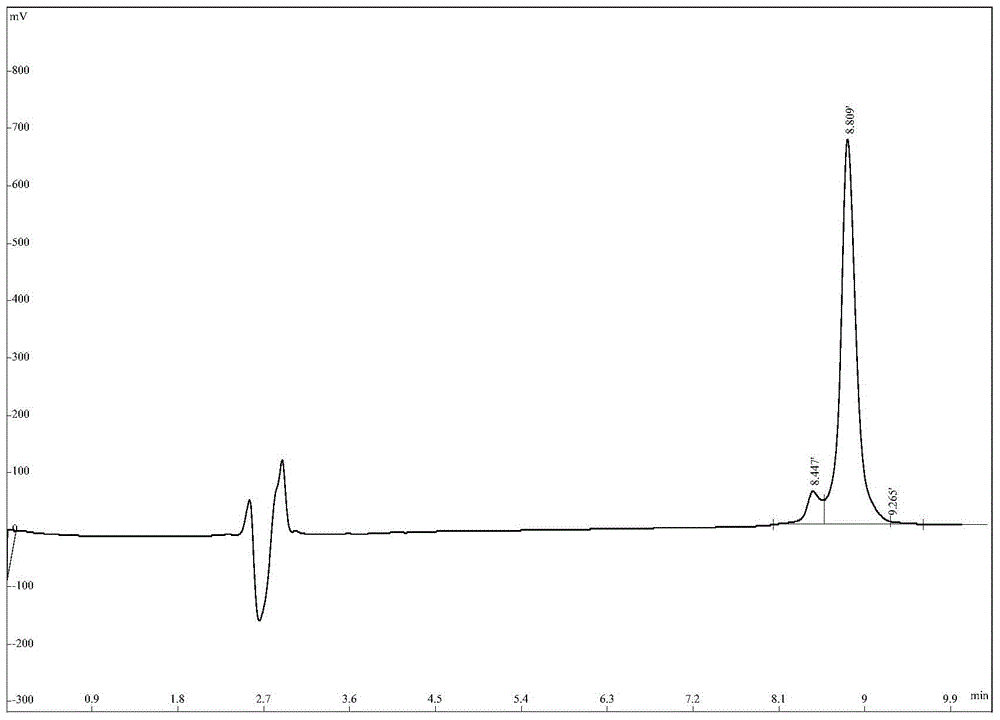
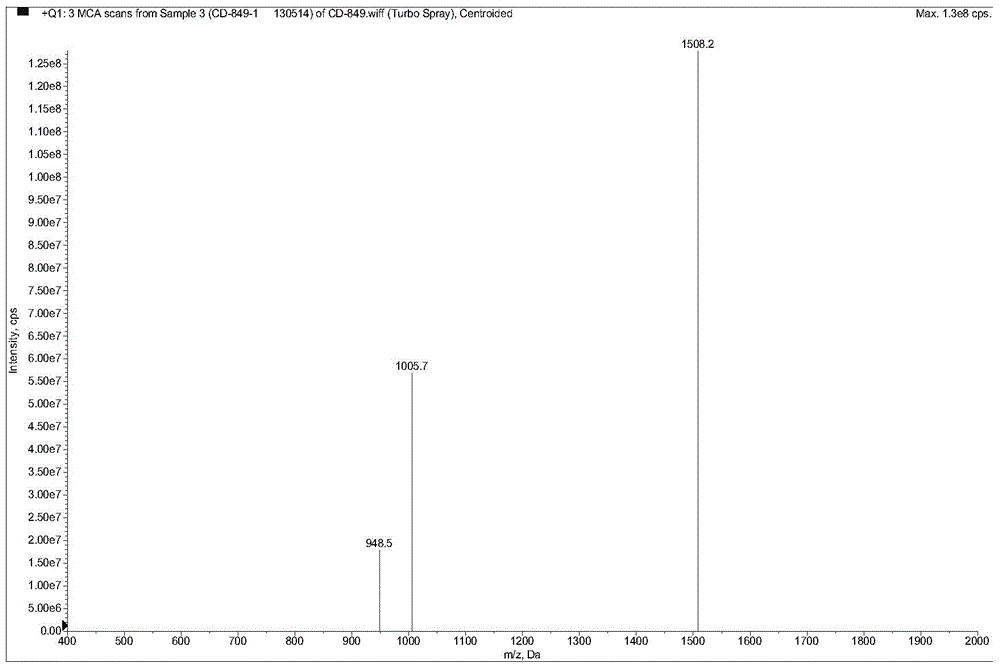
[0050] The HPLC spectrum of AMP2 is as follows image 3 As shown, the mass spectrum as Figure 4 shown. Depend on image 3 , 4 It can be seen that the purity and molecular weight of AMP2 both meet the design requirements.
Embodiment 3
[0052] The 199-234 AA sequence segment of SPLUNC1 protein was selected as the core region of the active peptide to prepare a novel antibacterial active peptide AMP3 (PIQGLLDSLTGILNKVLPHLVQGNVCPLVNHVLRGL).
[0053] The preparation method and steps of the novel antibacterial active peptide AMP3 are the same as the preparation method and steps of the novel antibacterial active peptide AMP1, refer to Example 1.
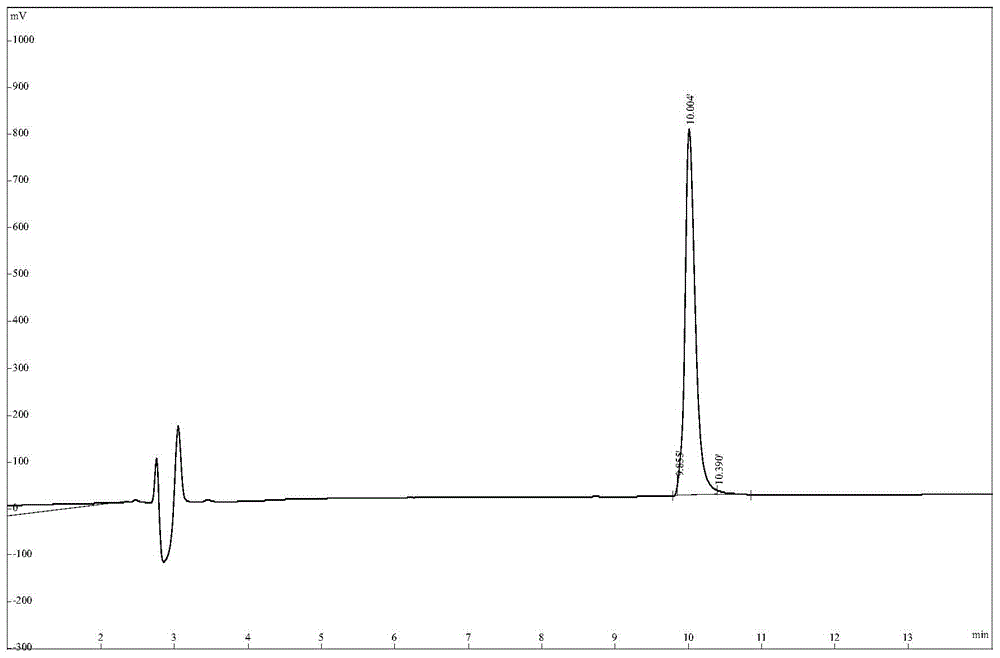
[0054] The HPLC spectrum of AMP3 is as follows Figure 5 As shown, the mass spectrum as Figure 6 shown. Depend on Figure 5 , 6 It can be seen that the purity and molecular mass of AMP3 both meet the design requirements.
[0055] The physical and chemical properties of the designed and prepared new antibacterial active peptide AMP1-AMP3 and SPLUNC1 protein are shown in Table 1. The physical and chemical properties of the designed and prepared new antibacterial active peptide AMP1-AMP3 are significantly different from those of SPLUNC1 protein. The appropriate peptide ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com