Supramolecular phase change material containing n-isopropylcyclohexylamine and its preparation and application
A technology of isopropylcyclohexylamine and phase change materials, which is applied in the field of phase change materials, can solve the problems of small phase change enthalpy and low phase change temperature, and achieve fast phase change speed, good crystal plasticity and high product purity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
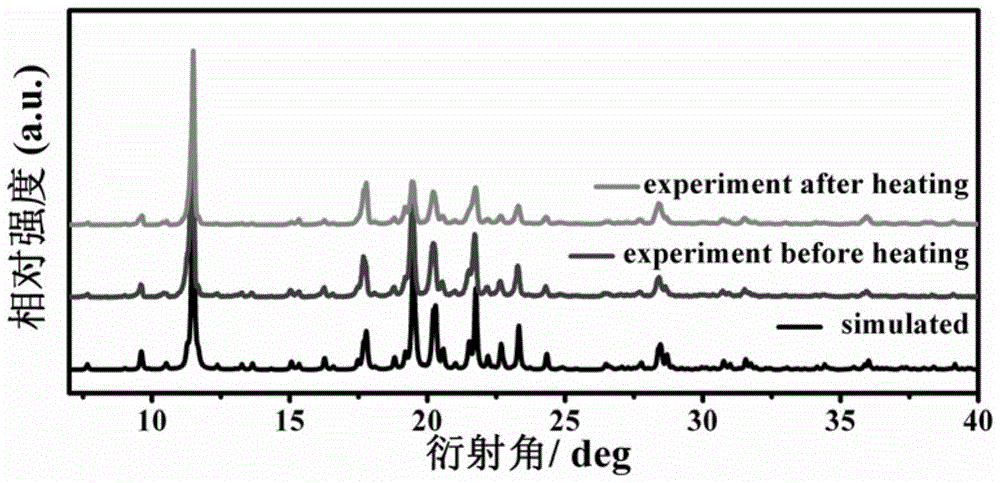
[0056] The preparation process of N-isopropylcyclohexylamine-trifluoroacetic acid supramolecular phase change material: 3mmol N-isopropylcyclohexylamine and 10mL deionized water (or deionized water / methanol volume ratio 1:1, deionized Deionized water / ethanol volume ratio 1:1, deionized water / ethyl acetate volume ratio 1:1, deionized water / acetone volume ratio 1:1) were mixed, and 3 mmol trifluoroacetic acid was added while stirring. The mixed solution was stirred for 10 min, filtered to obtain a clear solution, volatile and grown at normal temperature and pressure, and colorless transparent crystals were obtained after standing for ten days.
Embodiment 2
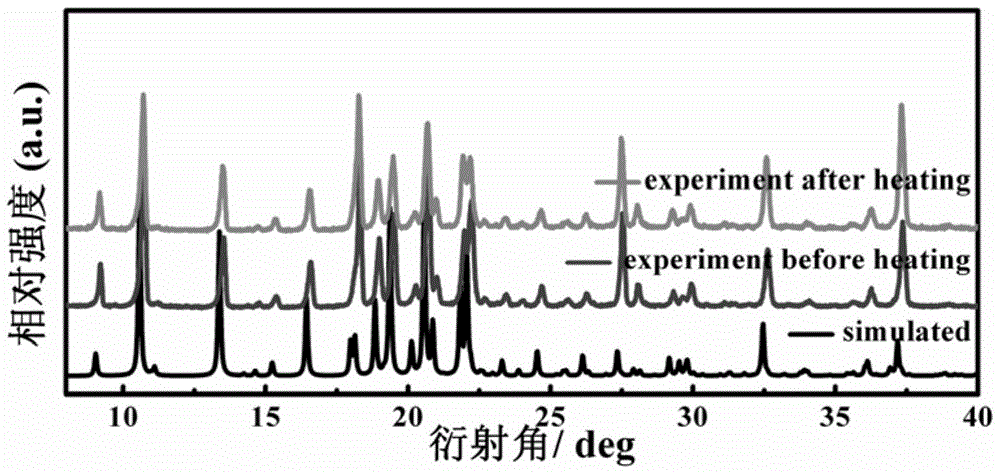
[0058] The preparation process of N-isopropylcyclohexylamine-trifluoromethanesulfonic acid supramolecular phase change material is as follows: 3mmol N-isopropylcyclohexylamine and 10mL deionized water (or deionized water / methanol volume ratio 1 : 1, deionized water / ethanol volume ratio 1:1, deionized water / ethyl acetate volume ratio 1:1, deionized water / acetone volume ratio 1:1) mix, add 3mmol trifluoromethyl sulfonic acid. The mixed solution was stirred for 10 min, filtered to obtain a clear solution, volatile and grown at normal temperature and pressure, and colorless transparent crystals were obtained after standing for two days.
Embodiment 3
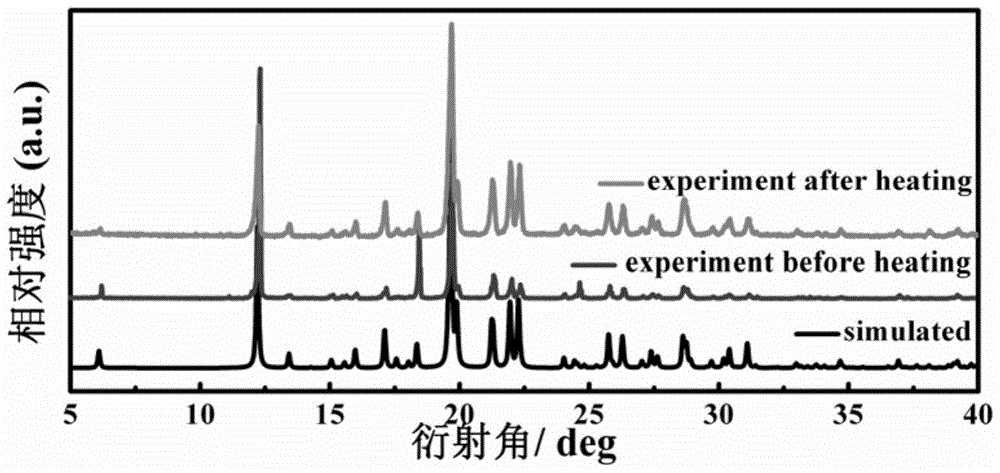
[0060] The preparation process of N-isopropylcyclohexylamine-perchloric acid supramolecular phase change material is: 3mmol N-isopropylcyclohexylamine and 10mL deionized water (or deionized water / methanol volume ratio 1: 1, Deionized water / ethanol volume ratio 1:1, deionized water / ethyl acetate volume ratio 1:1, deionized water / acetone volume ratio 1:1) were mixed, and 3mmol perchloric acid was added while stirring. The mixed solution was stirred for 10 min, filtered to obtain a clear solution, and volatilized and grown at normal temperature and pressure. After standing for a week, colorless transparent crystals were obtained.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com