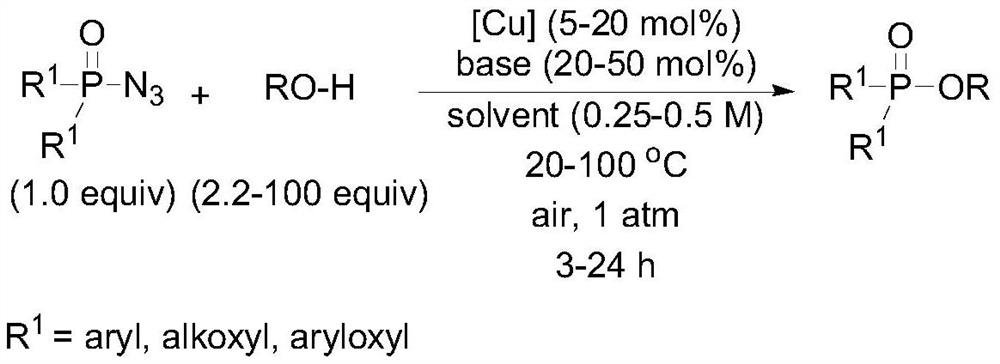
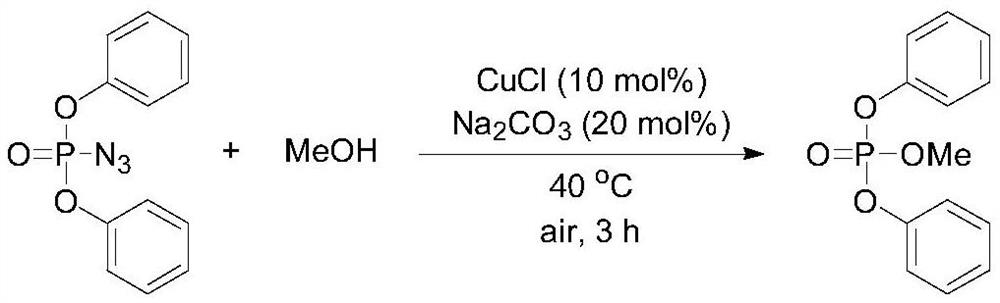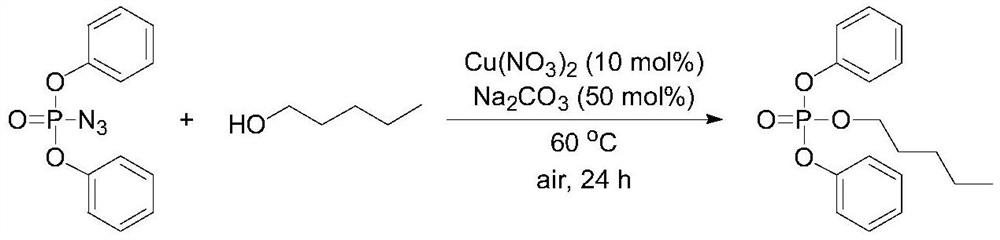A kind of synthetic method of copper-catalyzed phosphoric acid mixed ester compound
A synthesis method and compound technology are applied in the synthesis field of phosphoric acid mixed ester compounds, which can solve the problems of using toxic reagents, harsh reaction conditions, and high economic cost, and achieve the effects of convenient operation, high synthesis efficiency and broad application prospects.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037]
[0038] Synthesis of diphenyl methyl phosphate: Weigh 108 μL (0.5 mmol) of diphenyl phosphoric azide, 2 mL of methanol (49.4 mmol), cuprous chloride, sodium carbonate; diphenyl phosphoric azide and cuprous chloride The substance ratio of the substance is 1:0.1; the substance ratio of diphenylphosphoryl azide and sodium carbonate is 1:0.2; the weighed reactants are sequentially added to a 25mL reaction tube with a magnet, and the The mixture was stirred on a magnetic stirrer, then heated slowly to 40°C, and the progress of the reaction was monitored by thin-layer chromatography. Under 0.1MPa, stop the reaction after 3h and cool to room temperature. The mixture was diluted with water (5 mL), and extracted with ethyl acetate (10 mL×3), and the organic phases were combined. The organic phase was washed with saturated brine (5mL), dried by adding anhydrous sodium sulfate, concentrated by filtration, separated by column chromatography, using 300-400 mesh silica gel as th...
Embodiment 2
[0042]
[0043] Synthesis of diphenyl amyl phosphate: Weigh 108 μL (0.5 mmol) of diphenyl phosphate azide, 2 mL (18.4 mmol) of n-amyl alcohol, copper nitrate, sodium carbonate; diphenyl phosphate azide and copper nitrate The ratio of the amount of diphenylphosphoryl azide to sodium carbonate is 1:0.5; the weighed reactants are added to a 25mL reaction tube with a magnet in turn, and the Stir on a magnetic stirrer, then slowly heat to 60°C, and monitor the progress of the reaction by thin-layer chromatography. Under 0.1MPa, stop the reaction after 24h and cool to room temperature. The mixture was diluted with 0.1 mol / L hydrochloric acid (5 mL), and extracted with ethyl acetate (10 mL×3), and the organic phases were combined. The organic phase was washed with saturated brine (5mL), dried by adding anhydrous sodium sulfate, concentrated by filtration, separated by column chromatography, using 300-400 mesh silica gel as the stationary phase, and mixed with different proportion...
Embodiment 3
[0047]
[0048] Synthesis of 3-methylbutyl diphenyl phosphate: Weigh 108 μL (0.5 mmol) of diphenyl phosphoric azide, 0.2 mL (1.84 mmol) of isoamyl alcohol, copper chloride, sodium carbonate, and cyclohexane; The molar ratio of diphenyl phosphoric acid nitrogen to copper chloride is 1:0.1; the molar ratio of diphenyl phosphoric azide to sodium carbonate is 1:0.5; the volume ratio of cyclohexane to isoamyl alcohol is 9 : 1; Add the weighed reactants in turn to a 25mL reaction tube with a magnet, stir on a magnetic stirrer at room temperature, then slowly heat to 60°C, and monitor the progress of the reaction with thin-layer chromatography. Under 0.1MPa, stop the reaction after 24h and cool to room temperature. The mixture was diluted with 0.1 mol / L hydrochloric acid (5 mL), and extracted with ethyl acetate (10 mL×3), and the organic phases were combined. The organic phase was washed with saturated brine (5mL), dried by adding anhydrous sodium sulfate, concentrated by filtrat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com