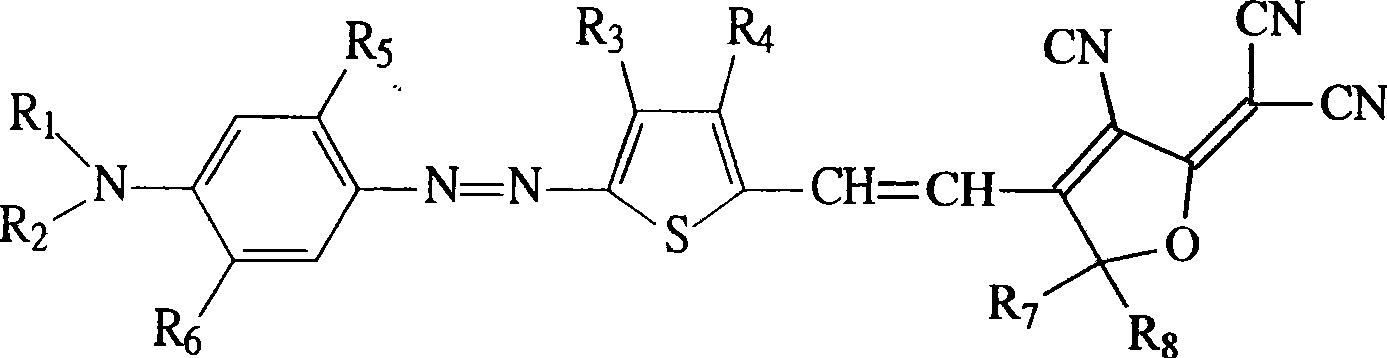
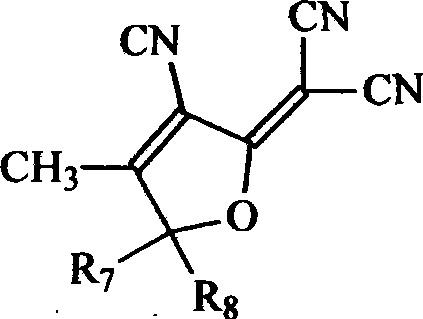
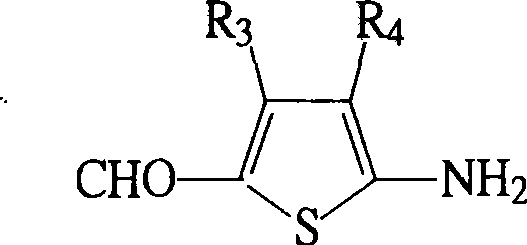
Second-order non-linear optical polymer containing azo and thiophene ring, and its synthesizing method and use
A second-order nonlinear, polymer technology, applied in the field of nonlinear optical materials, can solve problems such as stability degradation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] Synthesis of 3,4-dibutyl-5-amino-2-thiophenecarbaldehyde tin tetrachloride complex
[0055] 45 g (0.2 mol) of SnCl 2 2H 2 O was dissolved in 60ml of concentrated hydrochloric acid, when the system temperature dropped to 5°C, 17.8 grams (0.066mol) of 3,4-dibutyl-5-nitro-2-thiophenecarbaldehyde was added at one time, and stirred at 40°C for 1 hour to obtain Off-white solid suspension.
Embodiment 2
[0057] Synthesis of 3,4-dibutyl-5-amino-2-thiophenecarbaldehyde
[0058] The off-white solid suspension obtained in Example 1 was adjusted to alkaline with 40wt% NaOH aqueous solution, then extracted with ether, and the ether layer was washed with MgSO 4 After drying and evaporation, the obtained crude product can be directly used in the next reaction.
Embodiment 3
[0060] Synthesis of 2-[4-(N,N-dihydroxyethylamino)azophenyl]3,4-dibutyl-5-thiophenecarbaldehyde
[0061] The 3,4-dibutyl-5-amino-2-thiophenecarbaldehyde tin tetrachloride complex off-white solution obtained in Example 1; or the 3,4-dibutyl-5-amino obtained in Example 2 The hydrochloric acid solution of -2-thiophenecarbaldehyde was cooled to 2°C; 0.067 moles of NaNO 2 Dissolve in 15ml of water, and slowly add dropwise to the above gray-white solution or the hydrochloric acid solution of 3,4-dibutyl-5-amino-2-thiophenecarbaldehyde at below 5°C, and continue to stir for 1 hour to obtain a diazonium salt solution .
[0062] Dissolve 0.080mol of N, N-dihydroxyethylaniline in 10ml of 36% HOAC, cool to 3°C in an ice-salt bath, slowly add the above diazonium salt solution dropwise to the HOAC of N,N-dihydroxyethylaniline In the solution, the reaction temperature is controlled below 5°C, the pH value of the system is adjusted between 3 and 6 with NaOH, and the stirring is continued f...
PUM
| Property | Measurement | Unit |
|---|---|---|
| electro-optic coefficient | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com