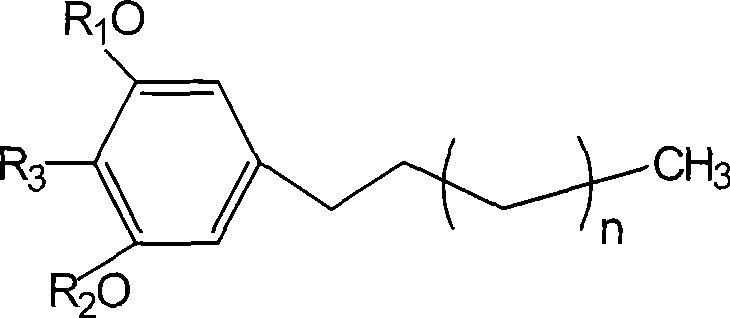
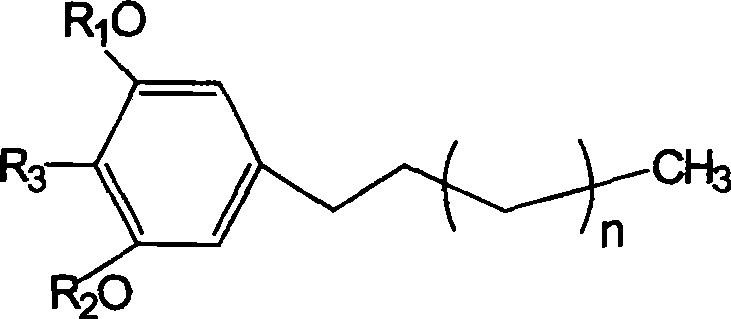
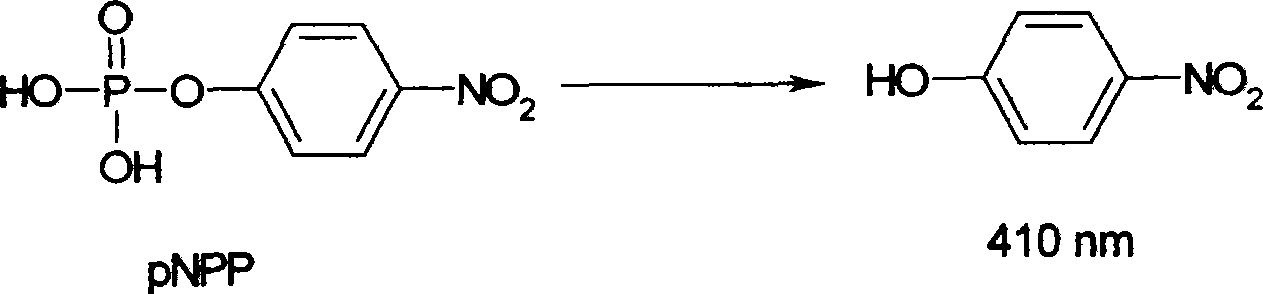Preparation and use of compounds i.e., 1,3-dihydroxy-5-alkyl benzene as inhbitor of protein-tyrosine-phosphatase 1B
A compound, the technology of alkylbenzene, applied in the field of medicine, can solve problems such as unseen
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Example 1: Preparation of compound 2-methyl-5-tridecyl-1,3-benzenediol (2-methyl-5-tridecyl-1,3-benzodiol)
[0032] Olive plum 3.0kg was leached three times with methanol, each time for one week, the methanol extract was combined, methanol was removed under reduced pressure, and then H 2 O was dissolved, then extracted three times with petroleum ether, ethyl acetate, and n-butanol respectively, and the ethyl acetate was combined and evaporated to dryness to obtain 28 g of extract.
[0033] Put 28g of ethyl acetate extract on a silica gel (100-200 mesh) column, elute with petroleum ether / ethyl acetate 95:5-50:50, 500ml / part, 100 parts in total, and combine after detection by thin layer chromatography There are 15 parts, and the fourth part is subjected to silica gel (200-300 mesh) column chromatography, eluted with petroleum ether / diethyl ether 90:10-50:50, and divided into F41 (500mg), F42 (850mg), F43 (350mg) three parts, F41 is subjected to Sephadex LH-20 gel column ...
Embodiment 2
[0035] Example 2: Preparation of compound 1,3-dihydroxy-5-undecylbenzene (1,3-dihydroxy-5-undecylbenzene)
[0036] Put 28g of ethyl acetate extract on a silica gel (100-200 mesh) column, elute with petroleum ether / ethyl acetate 95:5-50:50, 500ml / part, 100 parts in total, and combine after detection by thin layer chromatography There were 15 fractions, and the fifth fraction was subjected to silica gel (200-300 mesh) column chromatography, eluted with petroleum ether / ethyl acetate 90:10-50:50, 100ml / part, 90 parts in total. Thin-layer chromatography detection, developer petroleum ether / diethyl ether (1:1), combined into three parts, F51 (29-36, 2.0g), F52 (40-55, 500mg), F53 (65-85, 200mg ); F52 is subjected to Sephadex LH-20 gel column chromatography again, with CHCl 3 / MeOH (1:1) elution, 5ml / part, 30 parts in total, TLC detection, developer petroleum ether / diethyl ether (1:1), combined into F521 (10-19, 300mg) and F522 (25 -29, 90mg) two parts; F522 is through Sephadex LH-...
Embodiment 3
[0038] Example 3: Preparation of Compound 1 and Compound 2 Derivatives
[0039] (1) Preparation of compound 1 and compound 2 methylate
[0040] Weigh 5.0mg of compound 1 and compound 2 samples into a 10mL round-bottomed flask, add dissolved CH 2 N 2 Diethyl ether solution 2mL, evaporate ether and CH 2 N 2 Afterwards, the methylate of compound 1 (n=10, R 3 =CH 3 , R 1 , R 2 =CH 3 ) and the methylate of compound 2 (n=8, R 3 = H, R 1 , R 2 =CH 3 )
[0041] (2) Preparation of compound 1 and compound 2 acetylate
[0042] Weigh 5.0 mg of compound 1 and compound 2 samples into a 25 mL round bottom flask, add 1.5 mL of anhydrous pyridine and acetic anhydride, stir on a magnetic stirrer for 24 h, remove pyridine and acetic anhydride under reduced pressure, and obtain the acetyl compound (n=10, R 3 =CH 3 , R 1 , R 2 =Ac) and the acetylated compound of compound 2 (n=8, R 3 = H, R 1 , R 2 =Ac).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com