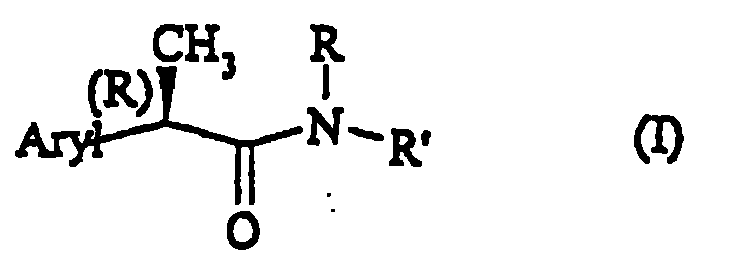
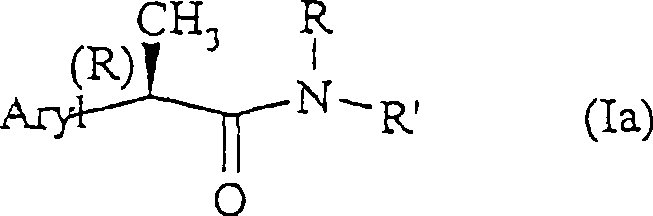
Amide for inhibition of IL-8-induced chemiotaxis of neutrophil leucocytes
A technology of propionamide and arylpropionamide, applied in the direction of amide active ingredients, non-central analgesics, medical preparations containing active ingredients, etc., can solve problems such as disease progression
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0208] (R, S')-2-[(4'-isobutyl)phenyl]-N-(2-carboxyethylpropionamide
[0209] To a solution of R(-) ibuprofen (5 g; 24.24 mmol) in DMF was added 3 g of 1-hydroxybenzothiazole (HOBT) (22.2 mmol) with stirring and cooled to about 0°C. After 15 minutes, a DMF solution (5 mL) of a mixture of L-alanine methyl ester hydrochloride (3.2 g; 22.2 mmol) and triethylamine (3 mL) was added; finally, a total of 5 g of DCC was continuously added in portions . The mixture was stirred at 0°C for 2 hours, then at room temperature overnight. After dicyclohexylurea precipitate was filtered off, the filtrate was diluted with ethyl acetate (50 mL). The organic phase was washed successively with 10% citric acid solution (2 x 20 mL), saturated sodium bicarbonate solution (2 x 20 mL) and saturated sodium chloride solution (20 mL). Dry over sodium sulfate, and evaporate the solvent under low pressure to obtain a residue (3.86 g), which is suspended in hexane (60 mL), stirred overnight, and a white c...
Embodiment 2
[0215] By the step of embodiment 1, L-alanine is changed into D-alanine methyl ester and glycine methyl ester, can make following compound:
[0216] (R,R')-2-[(4'-isobutyl)phenyl]-N-(2"-carboxyethyl)propionamide, which is a light butter, [α] D =+5 (c=0.5%; Methanol)
[0217] 1 H-NMR (CDCl 3): δ 7.20-7.07(m, 4H); 5.97(bs, CONH); 4.45(m, 1H); 3.60(m, 1H), 2.45(d, 2H, J=7Hz); 2.45(d, 2H, J = 7 Hz); 1.85 (m, 1H); 1.53 (d, 3H); 1.35 (d, 3H); 0.91 (d, 6H, J = 7 Hz).
[0218] R(-)-2-[(4'-isobutyl)phenyl]-N-carboxymethylpropionamide, melting point is 87-90°C;
[0219] 1 H-NMR (CDCl 3 ): δ 7.23-7.07(m, 4H); 5.93(bs, CONH); 4.13-3.93(m, 2H); 3.63(q, 1H, J1=8Hz, J2=15Hz), 2.45(d, 2H, J = 7 Hz); 1.87 (m, 1H); 1.53 (d, 3H, J = 7 Hz); 0.93 (d, 6H, J = 7 Hz).
Embodiment 3
[0221] (R)-N-[2′-(4”-isobutylphenyl)propionyl]-2-aminoacrylic acid
[0222] According to the steps of Example 1, (R, R')-2-[(4'-isobutyl)phenyl]-N-2"-(3"- Mercapto-carboxyethyl) propionamide. Under an inert atmosphere, a solution of 3 g of this compound in anhydrous dichloromethane was cooled to -10°C, and a 1M solution of boron tribromide in dichloromethane (6 mL) was added dropwise with stirring. The reaction mixture was stirred at -10°C for 1 hour and at room temperature for 6 hours. The resulting mixture was diluted with water (20 mL), the phases were separated and the aqueous phase was further extracted with dichloromethane. The combined organic phases were washed with saturated sodium bicarbonate solution (3 x 20 mL). The basic aqueous phase was acidified to pH=2 with 2N hydrochloric acid and extracted with dichloromethane (3 x 10 mL). The organic extracts were mixed, after drying over sodium sulfate and evaporating the solvent, a butter oil (R) N-[2'-(4"-isobutylphe...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com